������COCl
2�������ϡ��Ƹ��ҩ�ȹ�ҵ����������;����ҵ�ϲ���CO��Cl
2�ڻ���̿���ºϳɣ�
��1��ʵ�����г������Ʊ������Ļ�ѧ����ʽΪ
MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O
MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O
��
��2����ҵ��������Ȼ������Ҫ�ɷ�ΪCH
4����CO
2���и��������Ʊ�CO����֪CH
4��H
2��CO��ȼ���ȣ���H���ֱ�Ϊ-890.3kJ?mol
-1��-285.8kJ?mol
-1��-283.0kJ?mol
-1��������1m
3����״����CO��������Ϊ
5.52��103KJ
5.52��103KJ
��
��3��ʵ�����п����ȷ£�CHCl
3����˫��ˮֱ�ӷ�Ӧ�Ʊ��������䷴Ӧ�Ļ�ѧ����ʽΪ
CHCl3+H2O2=HCl+H2O+COCl2
CHCl3+H2O2=HCl+H2O+COCl2
��
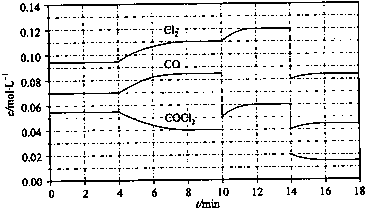
��4��COCl
2�ķֽⷴӦΪCOCl
2��g��=Cl
2��g��+CO��g����H=+108kJ?mol
-1����Ӧ��ϵ�ﵽƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ����10min��14min��Ũ�ȱ仯����δʾ������

�ټ��㷴Ӧ�ڵ�8minʱ��ƽ�ⳣ��K=
0.234mol?L-1
0.234mol?L-1
��
�ڱȽϵ�2min��Ӧ�¶�T��2�����8min��Ӧ�¶�T��8���ĸߵͣ�T��2��
��
��
T��8�������������������=����
����12minʱ��Ӧ��T��8�������´ﵽƽ�⣬���ʱc��COCl
2��=
0.031
0.031
mol?L
-1�ܱȽϲ���CO��2��3min��5��6min��12��13minʱƽ����Ӧ����[ƽ����Ӧ���ʷֱ���v��2��3����v��5��6����v��12��13����ʾ]�Ĵ�С
v��5��6����v��2��3��=v��12��13��
v��5��6����v��2��3��=v��12��13��
��
�ݱȽϷ�Ӧ��COCl
2��5��6min��15��16minʱƽ����Ӧ���ʵĴ�С��v��5��6��
��
��
v��15��16�������������������=������ԭ����
����ͬ�¶�ʱ���÷�Ӧ�ķ�Ӧ���Ũ��Խ�ߣ���Ӧ����Խ��
����ͬ�¶�ʱ���÷�Ӧ�ķ�Ӧ���Ũ��Խ�ߣ���Ӧ����Խ��
��