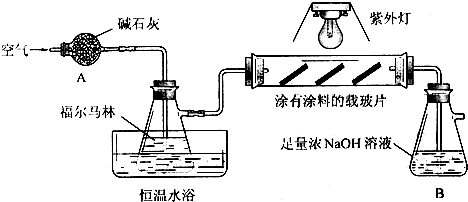
��2009?�Ͼ���ģ��ѡ���⣬������A��B���⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�����𣬲�����ѡ��Ŀ��Ӧ��ĸ��ķ���Ϳ�ڣ������ⶼ���𣬽���A�����֣�
A�����������з�Ӧ�ϳ������谷��
CaO+3C
CaC
2+CO�� CaC
2+N
2CaCN
2+C�� CaCN
2+2H
2O=NH
2CN+Ca��OH��
2��NH
2CN��ˮ��Ӧ��������[CO��NH
2��
2]�����غϳ������谷��
��1��д����Ca��ͬһ������������������ͬ���ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽ��
1s22s22p63s23p63d104s2��[Ar]3d104s2
1s22s22p63s23p63d104s2��[Ar]3d104s2
��CaCN
2��������ΪCN
22-����CN
22-��Ϊ�ȵ�����ķ�����N
2O��
CO2
CO2
���ѧʽ�����ɴ˿�����֪CN
22-���ӵĿռ乹��Ϊ
ֱ����
ֱ����
��
��2�����ط�����Cԭ�Ӳ�ȡ
sp2
sp2
�ӻ������ط��ӵĽṹ��ʽ��
������̼��ԭ��֮��Ĺ��ۼ���
C
C
������ĸ��
A��2���Ҽ� B��2���м� C��1���Ҽ���1���м�
��3�������谷��
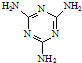
���׳ơ����������������������谷���������ᣨ
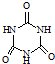
�������������������谷�����֮��ͨ��
���Ӽ����
���Ӽ����
��ϣ������������γɽ�ʯ��
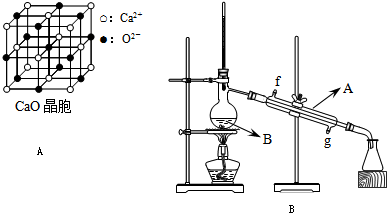
��4��CaO������ͼA��ʾ��CaO������Ca
2+����λ��Ϊ
6
6
��CaO�����NaCl����ľ����ֱܷ�Ϊ��CaO 3401kJ/mol��NaCl 786kJ/mol���������߾����ܲ������Ҫԭ����
CaO������Ca 2+��O 2-�Ĵ���������NaCl������Na+��Cl-�Ĵ�����
CaO������Ca 2+��O 2-�Ĵ���������NaCl������Na+��Cl-�Ĵ�����
��
B��ʵ������������������Ʊ��������������й����ʵ������������±�����ش��й����⣮
| ������ |
�ܶ�/g?cm-3 |
�е�/�� |
�ܽ��/100gˮ |
| ������ |
0.810 |
118.0 |
9 |
| ������ |
1.049 |
118.1 |
�� |
| ���������� |
0.882 |
126.1 |
0.7 |
�������������ֲ�Ʒ���Ʊ�
�ڸ����50mLԲ����ƿ�У�װ���ʯ������11.5mL��������9.4mL�����ᣬ�ټ�3��4��Ũ���ᣮȻ��װ��ˮ�������ã�ʵ������в��Ϸ����ȥ��Ӧ���ɵ�ˮ�����¶ȼƼ����������ܣ���������������Ӧ��
��1����ʵ������п��ܲ��������л������д���������ֵĽṹ��ʽ��
CH3CH2CH2CH2OCH2CH2CH2CH3
CH3CH2CH2CH2OCH2CH2CH2CH3
��
CH2=CHCH2CH3
CH2=CHCH2CH3
��
��2��ʵ����Ϊ����������������IJ��ʣ���ȡ�Ĵ�ʩ�ǣ�
�÷�ˮ����ʱ���߷�Ӧ���ɵ�ˮ�������������Ũ�ȣ�
�÷�ˮ����ʱ���߷�Ӧ���ɵ�ˮ�������������Ũ�ȣ�
��
ʹ�ù������ᣬ�����������ת����
ʹ�ù������ᣬ�����������ת����
��
�������������ֲ�Ʒ���Ʊ�
��1���������������ֲ�Ʒ�����µIJ������о��ƣ���ˮϴ ������ ������ˮMgSO
4���� ����10%̼����ϴ�ӣ���ȷ�IJ���������
C
C
������ĸ����
A���٢ڢۢ�B���ۢ٢ܢ�C���٢ܢ٢ۢ�D���ܢ٢ۢڢ�
��2�������������ͼB��ʾװ������
1����ͼ������A������
������
������
����ȴˮ��
��
��
�ڽ��루����ĸ����
2���������ռ�������������Ʒʱ��Ӧ���¶ȿ�����
126.1��
126.1��
���ң�
�������
������ˮ��������������������Ӧ���ɵ�ˮ���Ϊ1.8mL����������ȡ���������������з�Ӧ���������û����ʧ���Һ��Ը���Ӧ�����������������IJ���
79.4%
79.4%
��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�



 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ  �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��
 +HNO3��Ũ��
+HNO3��Ũ��
 +HNO3��Ũ��
+HNO3��Ũ��

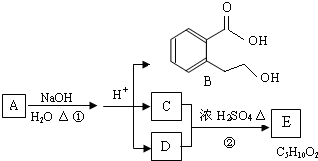


 д������֮һ����
д������֮һ���� д������֮һ����
д������֮һ����