
�������ֶ���������Ԫ��A��B��C�� D��E����ԭ��������������Aԭ��Լռ������ԭ��������88.6%��A+�ֳ�Ϊ���ӣ�B���γɻ�������������Ԫ�أ�CԪ�ص�����⻯��Y��ˮ��Һ�Լ��ԣ�E�Ƕ�����Ԫ���е縺����С��Ԫ�ء�A��B��C��E����Ԫ�ض�����DԪ���γ�ԭ�Ӹ����Ȳ���ͬ�ij���������Իش��������⣺
��1��д��A��E��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ____��
��2�����Ȼ�������Һ�μӹ�����E������������Ӧˮ�������Һ��������____��
��3��Y��Һ�Լ��Ե�ԭ����(��һ�����ӷ���ʽ��ʾ��____��
��4����������β���к��еĻ�����BD�ķ����ǣ�������PdC12��Һ��ͨA����β���������ɺ�ɫ������Pd����֤������β���к���BD��д����Ӧ�����ӷ���ʽ____��
��5�������й��������ʵıȽ��У�����ȷ���� ��
a�����ȶ��ԣ�H2S>SiH4 b�����Ӱ뾶��Na+>S2��
c����һ������N>O d��Ԫ�ص縺�ԣ�C>H
��6����֪����CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H=+49.0kJ/mol
��CH3OH(g)+3/2O2(g)=CO2(g)+2H2O(g) ��H=��192��9kJ/mol
����������ʽ��֪��CH3OH��ȼ����____������ڡ��������ڡ���С�ڡ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
��14�֣�ÿ��2�֣�
��1��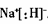
��2�����а�ɫ������Ѹ�ٱ�Ϊ����ɫ������ɺ��ɫ
��3��NH3+H2O NH4++OH-
NH4++OH-
��4��CO+PdCl2+H2O=Pd+CO2+2H++2Cl-
��5��b
��6�����ڣ�H2(g)+ O2(g)=H2O(l) ��H=-124.6KJ/mol
O2(g)=H2O(l) ��H=-124.6KJ/mol
��������������������������֪A��B��C��D��E�ֱ�ΪH��C��N��O��Na��
��1��Na��H�γ������ͻ�����
��2������������������NaoH��Ϻ����ɰ�ɫ������������������������������
��3��һˮ�ϰ����뷽��ʽ
��4��������ԭ��Ӧ����ʽ��д�����жϷ�Ӧ��������Ȼ����ƽ��
��5��Ԫ�������ɿ�֪���ǽ�����Խǿ����̬�⻯���¶���Խǿ��a��ȷ�����Ӳ���Խ�࣬�뾶Խ��S2->Na+��b����NΪ���������һ�����ܴ���O��c��ȷ���ǽ�����Խǿ���縺��Խ��d��ȷ��
��6���ɢڷ�Ӧ��ˮ����̬��Һ̬���ȿ�֪��CH3OH��ȼ���ȴ���192.9KJ���ɸ�˹���ɿ�֪��������ȼ���ȣ���д���Ȼ�ѧ����ʽ��
���㣺�������ʽṹ���֪ʶ���漰ԭ�ӽṹ��Ԫ�������ɡ�Ԫ�ؼ����������ʣ��Լ�ȼ���ȵļ��㣬�Ȼ�ѧ����ʽ����д�ȡ�


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ij����������Ԫ�ص�ԭ��M������һ����������Dz�,����ԭ�ӵ��������� ___________��д��������Χ�����Ų�ͼ___________��
��2��VIA���.��(Se).�ڻ������г����ֳ���������̬, H2SeO4��H2SeO3����_-___( ��ǿ����)��H2Se�����Ա�H2S__________(�ǿ��������)��
��3������A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ���������й�˵������ȷ���� ( )
| A��NH5�м������Ӽ����й��ۼ� |
| B��NH5���ۡ��е����NH3 |
| C��NH5����Ͷ������ˮ�У��ɲ����������� |
| D��0.1 mol NH5���5 mol N��H�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F�ܱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| A | A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�� |
| B | BԪ�ص�ԭ�Ӽ۵����Ų�Ϊns11np14 |
| C | M�Ļ�̬ԭ��L���������K���������3�� |
| D | D�ǵ��������е�һ��������С��Ԫ�� |
| E | E�ǵؿ��к������Ľ���Ԫ�� |
| F | �ж��ֻ��ϼۣ���ij�ָ������ӵļ۵��Ӿ��н��ȶ��İ�����ṹ |
 ����
���� �������֮��Ϊ ��
�������֮��Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������B������������������������ȡ���д���пհס�
| | | A | |
| B | C | | D |
| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����H2�����ʵ��� | ��������ʵ��� |
| y | �����ʵ��� | ƽ�ⳣ��K | ��ת���� | ���������ʵ����ܺ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�����������Ԫ�ص������Ϣ������w��X��Y��ZΪ������Ԫ�أ�ԭ���������ε�����
| Ԫ�� | �����Ϣ |
| W | ����Ϊ�ܶ���С������ |
| X | Ԫ����������������֮��Ϊ0 |
| Y | ��ҵ��ͨ������Һ̬��������䵥�ʣ��õ��ʵ�ij��ͬ���������DZ�������ر���������Ҫ���� |
| Z | ����������Ϊ23��������Ϊ12�ĺ��� |
| T | ��������Ϊ��ɫ�������Ժã������岻��ȱ�ٵĻ���Ԫ�أ���ɫ��Ӧʱ����Ϊ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CaC2�����ڹ̵���CaC2��N2  CaCN2��C��CaCN2(�谱����)��ˮ��Ӧ������NH3��
CaCN2��C��CaCN2(�谱����)��ˮ��Ӧ������NH3��
��1��д����Ca��ͬһ������������������ͬ���ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽ�� ��
��2��C��N��O����Ԫ�صĵ�һ��������С�����˳���� ��
��3��NH3��Nԭ�ӵ��ӻ���ʽΪ �����ݼ۲���ӶԻ��������Ʋ�CO32���Ŀռ乹��Ϊ ��
��4��CaCN2��������ΪCN22������CN22����Ϊ�ȵ�����ķ����� ����дһ�ֻ�ѧʽ���ɣ���д��CaCN2ˮ�ⷴӦ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֡���ش��������⣺
����Ԫ�������ڱ��е����λ�ÿ�֪�١���ֱ���H��C��N��O��Mg��Al��Cl��Ca��Mn��Fe��
��1������Ԫ���У�����s������____________(��Ԫ�ط���)��
��2��д��Ԫ�آܵĻ�̬ԭ�ӵļ۵����Ų�ͼ____________________��
��3��Ԫ�ص�һ������Ϊ��________�� (����ڡ���С�ڡ�)��
��4��Ԫ�آ���̬�⻯���VSEPRģ��Ϊ________���÷���Ϊ________����(����ԡ��Ǽ��ԡ�)��������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ_____________________________��
��5�����ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
����֪��ԭ�Ӱ뾶Ϊdcm��NA���������ӵ�������Ԫ�آ����ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ ,�þ�����ܶ�Ϊ ������ĸ��ʾ��
��6��ʵ��֤�����ݺ͢�������KCl��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ�����ͼ��ʾ������֪3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�м���Ԫ�ص����ĺ�����Ӳ�ṹ��ͼ��ʾ��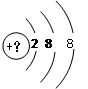
��ش��������⡣
��1�������������������������ķ�����_____________��
��2��������������Һ��ʹ��ˮ��ɫ�������ֻ��ǣ��������ķ�����____________��
��3���������������Ժ������õ�1�����Ӻ��Ϊԭ�ӣ��䵥�ʵĻ�ԭ�Ժ�ǿ���������ķ�����________________��
��4���������Ļ�ԭ�Ժ�����ʧȥ1�����Ӻ��Ϊԭ�ӣ��䵥�ʵ������Ժ�ǿ���������ķ�����_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��16�֣���֪A��B��C��D��E��F��G��H����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ���������������ڲ��������������C�Ļ�̬ԭ�Ӻ�����7�ֲ�ͬ�˶�״̬�ĵ��ӣ�E�ǵ縺������Ԫ�أ�F���⻯�������������Ӧ��ˮ�����Ϊǿ�ᣬG��H�ֱ������ڱ���1��18�����еĵ�10��12����Ԫ�ء���ش��������⣺
��1��DԪ�������ڱ�λ���� ��G�ĺ���۲�����Ų�ʽΪ ��
��2��E��A�γɵĻ������F��A�γɵĻ�����ķе� ����ߡ��͡�������ԭ���� ��
��3��B��Cԭ�ӵĵ�һ�����ܽϴ���� ����Ԫ�ط��ţ�����ԭ���� ��
��4��BD32��������Bԭ�Ӳ�ȡ �ӻ�����дһ����BD��Ϊ�ȵ�����ķ��ӵĵ���ʽ ��
��5��Ԫ��H��һ�����ᄃ��ľ����ṹ��ͼ��ʾ��������Ļ�ѧʽ�� ��Ԫ��H��������������ڰ�ˮ�У����ɺ�ͭ����������ͬ��λ�������ӣ�д���÷�Ӧ�����ӷ���ʽΪ ��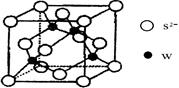
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com