
������Ҫ�Ļ���ԭ�� ���ɷ������з�Ӧ���ɢ�͢���
���ɷ������з�Ӧ���ɢ�͢���
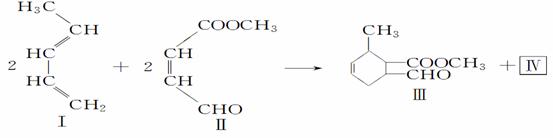
 |
(1)a�Ľṹ��ʽ��________________���ڵķ�Ӧ������____________��
(2)�ü״���ij�л����������Ӧ�ɺϳɻ������д���÷�Ӧ�Ļ�ѧ����ʽ______________________________________________________________��
(3)������������Ƶ�������ͭ����Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ__________��
(4)��������Ǣ��ͬ���칹 �壬Ҳ��ͬ������Ԫ�������Ľṹ��ʽΪ_____��
�壬Ҳ��ͬ������Ԫ�������Ľṹ��ʽΪ_____��

(1)A��C�Ľṹ��ʽ�ֱ���_____________��__________��
(2)����������,C������NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��____________��
(3)E��һ����Է���������AС14�ķ����ᡣд����������������E������ͬ���칹��Ľṹ��ʽ:____________________________________________��
���ܷ���������Ӧ
��һ�������¿ɷ���ˮ�ⷴӦ
�۷��ӵĺ˴Ź����������������]
(4)F��B�ڼ���Һ��ˮ������ữ�IJ��F��һ�������¿ɾۺϳɸ߷��ӻ�����,д���÷�Ӧ�Ļ�ѧ����ʽ: _____________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӻ��������ȡ��ķ����ǣ�������ҽ�ȡҺ��ͨ��Cl2���������ķ������û��ĵ��ᴿ������ȡҺ��ͨ��Cl2ʱ��������������������Cl2��ICl��IBr��Ϊ���������������ʣ�ʹ���еĵ���ȫ����������ɼ���������(����)
A��CaO
B��KI
C��H2O
D���ƾ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ���ڱ�¶�ڿ����л���ʵ��� ���� ����
A.CuSO4��Һ B.Na2SiO3��Һ C.FeSO4��Һ D.NaCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������̬���Ļ������0.1mol���ڿ�������ȫȼ�յõ�0.16molCO2��3.6gˮ������˵����ȷ���ǣ� ��
A��һ�������飬�������� B��һ�������飬��������
C��һ���Ǽ������ϩ�Ļ���� D��һ�������飬��������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�����������˵����ȷ����
A���ú�0.1mol FeCl3����Һ��������ˮ��Ӧ�Ƶõ�Fe(OH)3�����н�����Ϊ0.1NA
B��46g NO2��N2O4�Ļ�������к�Nԭ������ΪNA
C����״���£�5.6L CCl4���еķ�����Ϊ0.25NA
D�������ʵ�����NH4����OH������������Ϊ10NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ȷ��ij�����Ƿ���ʣ���ѡ�Լ������������ʣ��������
A��Na2SO3�Ƿ�������BaCl2�� B��FeCl2�Ƿ�������KSCN��
C��KI�Ƿ�������������Һ�� D����ˮ�Ƿ�ʧЧ��pH��ֽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����( )��
A��Na2O��Na2O2���Ԫ����ͬ������CO2��Ӧ�IJ��ﲻͬ
B��2Na2O2+2H2O==4NaOH+O2�� Na2O2��������H2O�ǻ�ԭ��
C��Na2O2����ˮ����O2�����ӷ���ʽΪ��Na2O2+H2O==2Na++2OH��ʮO2��
D��Na2O2����������ߵĹ�����ʱ��Ԫ�ؼ��������ֱ���ԭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��3.9gNa2SO4��Na2CO3�Ļ��������ˮ�õ���ҺA����A�м���������һ��Ũ�ȵ�BaCl2��Һ20.0mL��Ȼ����˵ó���B����ҺC.��C�м�����AgNO3��Һ��������11.48g��������B�м�������ϡ���ᣬ��������0.36g.���㣺
(1)�Ȼ�����Һ�����ʵ���Ũ�ȣ�
(2)ԭ������������Ƶ�����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com