
��1����ѧʵ�����ע�ⰲȫ�������������ڰ�ȫ��������___________��ѡ����ĸ����
A.������ԭ����ͭʵ���У��ȼ�������ͭ��ͨ����
B.����ʯ��ʱ������һ��ʱ�����δ�����Ƭ�����̰ο���Ƥ����Ͷ�����Ƭ
C.ʵ�������Ƶ�ʵ��ʱ�����µ���мͶ�뵽��Һ����
D.����Ũ������ƾ����Һʱ����1����ľƾ�����3�����Ũ������
E.Ƥ����մ������Ũ����ʱ�������ô���ˮ��ϴ����Ϳ��ϡ̼��������Һ
F.����ϩʱ��������Ϊ300 ����¶ȼƴ�������Ϊ200 ����¶ȼƣ��ⷴӦҺ���¶�
��2����ӵ������ⶨ������ͭ������ԭ���ͷ������£�
��֪���������������£�������Cu2+��I-���ö�������I2��I2���ڹ�����KI��Һ�У�
I2+I-====![]() ����֪�����ԣ�Fe3+��Cu2+��I2��
����֪�����ԣ�Fe3+��Cu2+��I2��![]() ��
��
������I2����c mol��L-1 Na2S2O3����Һ�ζ���2![]() +
+![]() ====
====![]() +3I-��
+3I-��
ȷ��ȡa g��������������250 mL����ƿ����ĥ��������ƿ���У���50 mL����ˮ��5 mL 3 mol��L-1 H2SO4��Һ��������NaF���ټ���������10% KI��Һ��ҡ�ȡ����ϵ���ƿƿ�ǣ����ڰ���5 min����ַ�Ӧ����1��2 mL 05%�ĵ�����Һ����Na2S2O3����Һ�ζ�����ɫ��ȥʱ������ȥV mL��Һ��
��ʵ���У��ڼ�KIǰ���������NaF���Ʋ������ÿ����ǣ�______________________��
��ʵ���м���5 mL 3 mol��L-1 H2SO4��Һ������Ϊ����������ǣ�_______________________��
�۱�ʵ�����õ���ƿ��������ͨ��ƿ����Ϊ��_____________________________________��
������ͭ��Һ��⻯����Һ��Ӧ���ɰ�ɫ�������⻯��ͭ���������⣬�÷�Ӧ�����ӷ���ʽΪ��____________________________________________________________________��
�ݸ��ݱ���ʵ�������õ���������ͭԪ�ص���������Ϊw(Cu)=______________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
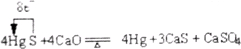
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(a)A��B��C��D��E����Ԫ�ص�ԭ����������������A��Dͬ���壬C��Eͬ���壬B��Cͬ���ڡ�
(b)W��A��B��ɣ�������ԭ�Ӹ�����ΪA��B=1��1������ʱΪ��̬��
(c)X��A��C��ɣ�������ԭ�Ӹ�����ΪA��B=2��1������ʱΪҺ̬��
(d)Y����C��D�γɵ����ӻ������Y����ӦԪ�ص���֮��Ϊ��C��D=1��1��
(e)Z����D��E�γɵ����ӻ�������������ӱ���������һ�����Ӳ㣬���������Ӹ�����Ϊ1��2����������и�С�⣺
(1)��Ԫ�ط��ţ�A_____��B_____��C_____��D_____��E_____��
(2)W�Ļ�ѧʽ��__________��
(3)X�Ľṹʽ��__________��
(4)Y�ĵ���ʽ��__________��
(5)Z�Ļ�ѧʽ��__________��
(6)д�����з�Ӧ��ѧ����ʽ��Y��ˮ��Ӧ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ֻ�����W��X��Y��Z�����Ƕ����ɶ�����Ԫ��A��B��C��D��E�е�����Ԫ����ɵġ���֪��
(a)A��B��C��D��E����Ԫ�ص�ԭ����������������A��Dͬ���壬C��Eͬ���壬B��Cͬ���ڡ�
(b)W��A��B��ɣ�������ԭ�Ӹ�����ΪA��B=1��1������ʱΪ��̬��
(c)X��A��C��ɣ�������ԭ�Ӹ�����ΪA��B=2��1������ʱΪҺ̬��
(d)Y����C��D�γɵ����ӻ������Y����ӦԪ�ص���֮��Ϊ��C��D=1��1��
(e)Z����D��E�γɵ����ӻ�������������ӱ���������һ�����Ӳ㣬���������Ӹ�����Ϊ1��2����������и�С�⣺
(1)��Ԫ�ط��ţ�A_____��B_____��C_____��D_____��E_____��
(2)W�Ļ�ѧʽ��__________��
(3)X�Ľṹʽ��__________��
(4)Y�ĵ���ʽ��__________��
(5)Z�Ļ�ѧʽ��__________��
(6)д�����з�Ӧ��ѧ����ʽ��Y��ˮ��Ӧ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��1��C1��ѧ��ָ��һ��̼ԭ�ӵĻ������CH4��CO��CO2��CH3OH��HCHO�ȣ������ϳɸ��ֻ�ѧƷ�ļ�������ú����Ȼ���ƺϳ����ٽ�һ���Ʊ����ֻ�����Ʒ�ͽྻȼ�ϣ��ѳ�Ϊ����ѧ��ҵ��չ�ı�Ȼ���ơ����м״���C1��ѧ�Ļ�����
��CO��H2��һ�������������Ҷ�������n(CO)/n(H2)=_____________�������֣���
��������ƽ�������CmHn��ʾ����ϳ����Ϳ���n(CO)/n(H2)=(��m��n��ʾ)��
�ۼ״���һ����������CO��H2���������л���A��A�����Ӿۿ����ɸ߷���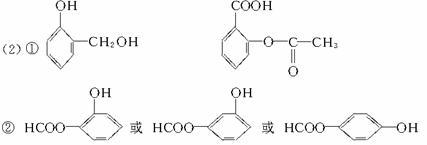 д������A�Ļ�ѧ����ʽ��_________________________________��
д������A�Ļ�ѧ����ʽ��_________________________________��
��2����֪��ȩ��һ���������ܷ������Ϸ�Ӧ��ʾ�����£�
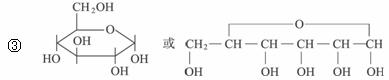
��֪��
��1827�����Ǿͷ����л���A�����ķ���ʽΪC13H18O7����һ����ˮ���ã�ˮ������B��C��
��B�ܷ���������Ӧ��BҲ���ɵ���ˮ��õ���B�ķ���ʽΪC6H12O6��
��C���Ȼ�����Һ�ܷ�����ɫ��Ӧ��1 mol C�������Ʒ�Ӧ�ɲ���1 mol H2��
��C���ʵ������������ʵ��������������ɵ�D��D�ķ���ʽΪC7H6O3����Է�������D��C��14��
��D������ȡ�����������Ǽ�λ������Br2�ڴ��������·���һ��ȡ�������������֣�D����̼��������Һ��Ӧ��
��D����������(CH3CO)2O����Ӧ���ɵó���ҩ��E�����ᣬE����̼�����Ʒ�Ӧ�ų�������̼��
�Իش��������⣺
��д���ṹ��ʽ��C_________________��E_________________��
��д����D��Ϊͬ���칹�壬���б����Һ������ṹ�Ľṹ��ʽ��_________________��ֻ��дһ�֣���
��Bͨ������Ԫ��״�ṹ���ڣ�д��B�Ļ�״�ṹ��ʽ��_________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com