
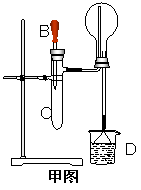 �ش�ʵ������ȡ���������а�������ʵ����������⣬����Ҫ����д�հ�
�ش�ʵ������ȡ���������а�������ʵ����������⣬����Ҫ����д�հ�
 |
|||
|
|||
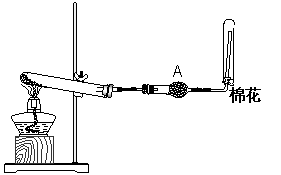
��1��ʵ������ͼ1��ʾװ���Ʊ������NH3 ��
�ٷ�Ӧ�Ļ�ѧ����ʽΪ�� ��װ�����ռ�NH3���Թܿڷ������������� ��
��Aװ�õ������� ��װ���ҩƷ�� ��
��2��ͼ2��ij��ѧС���Ʊ�NH3����������ʵ��ʱ�ĸĽ�װ�á���ͼ�װ�������װ�ã���ȡ2g�����Ȼ��װ���Թܵײ����ٿ��ٳ�ȡ2g�������Ƹ������Ȼ���Ϸ��������ô��еιܵ������������ι�Ԥ������Լ2mlŨ��ˮ�����ձ���ʢ���з�̪�Թܵ�ˮ����Ũ��ˮ�����Թ�������۲쵽�Թ��ڷ������ҷ�Ӧ���д������ݲ�����
��ͼ���е�NH4Cl��NaOH���������ܷ���CaO������棿 ����ܡ����ܡ� ��
������ж�ͼ����ƿ���ռ���������
��ͼ�����ռ���NH3��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�����������2mL H2O����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH3��ij�����ʡ�
��ͼ�����潺ͷ�ι��е�ˮ������ƿ�У��۲쵽��������
��˵������ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
| ��� | ʵ����� | ʵ������ |
| �� | ϡ�����м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ϡ�����м�����������Һ | �����Ա仯 |
| �ټ���õ���a | ����ɫ���ݣ���Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
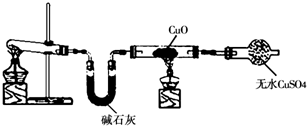
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ��������
��ش�
��1����ȡ�����Ļ�ѧ����ʽ�� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ �� �ڼ�ʯ�ҵ������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
|
��� |
ʵ����� |
ʵ������ |
|
�� |
ϡ�����м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
ϡ�����м�����������Һ |
�����Ա仯 |
|
�ټ���õ���a |
����ɫ���ݣ���Һ���� |
��ʵ��I��II��III��Ŀ���� ��
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ�� ��
�۽��õ���a���뵽��������Ĺ���������Һ�У���Һ�����ɫ���÷�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ�����и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
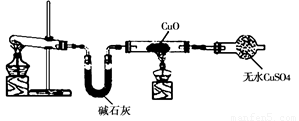
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ����������ش�
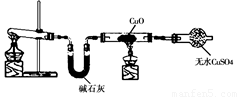
��1����ȡ�����Ļ�ѧ����ʽ�� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
�ڼ�ʯ�ҵ������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
|
��� |
ʵ����� |
ʵ������ |
|
�� |
ϡ�����м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
ϡ�����м�����������Һ |
�����Ա仯 |
|
�ټ���õ���a |
����ɫ���ݣ���Һ���� |
��ʵ��I��II��III��Ŀ���� ��
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 �ش�ʵ������ȡ���������а�������ʵ����������⣬����Ҫ����д�հ�
�ش�ʵ������ȡ���������а�������ʵ����������⣬����Ҫ����д�հ�
 | |||
| |||
��1��ʵ������ͼ1��ʾװ���Ʊ������NH3��
�ٷ�Ӧ�Ļ�ѧ����ʽΪ�� ��װ�����ռ�NH3���Թܿڷ������������� ��
��Aװ�õ������� ��װ���ҩƷ�� ��
��2��ͼ2��ij��ѧС���Ʊ�NH3����������ʵ��ʱ�ĸĽ�װ�á���ͼ�װ�������װ�ã���ȡ2g�����Ȼ��װ���Թܵײ����ٿ��ٳ�ȡ2g�������Ƹ������Ȼ���Ϸ��������ô��еιܵ������������ι�Ԥ������Լ2mlŨ��ˮ�����ձ���ʢ���з�̪�Թܵ�ˮ����Ũ��ˮ�����Թ�������۲쵽�Թ��ڷ������ҷ�Ӧ���д������ݲ�����
��ͼ���е�NH4Cl��NaOH���������ܷ���CaO������棿 ����ܡ����ܡ���
������ж�ͼ����ƿ���ռ���������
��ͼ�����ռ���NH3��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�����������2mLH2O����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH3��ij�����ʡ�
��ͼ�����潺ͷ�ι��е�ˮ������ƿ�У��۲쵽��������
��˵������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com