
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮
����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


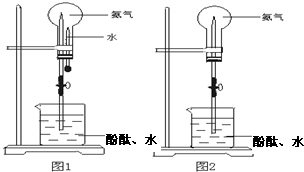
ͼ1 ͼ2
(1)д��ʵ������ȡ�����Ļ�ѧ����ʽ______________________________��
(2)�ռ�����Ӧʹ��__________����Ҫ�õ�����İ�����ѡ��__________���������
(3)��ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����__________��
��ʵ���ԭ����__________________________________________________��
(4)���ֻ�ṩ��ͼ2��װ�ã���˵��������Ȫ�ķ�����
____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
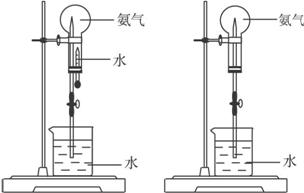
ͼ1 ͼ2
ͼ8-4
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ��___________________________________��
��2���ռ�����Ӧʹ��____________����Ҫ�õ�����İ�����ѡ��____________���������
��3����ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����__________����ʵ���ԭ����_________________________________________��
��4�����ֻ�ṩ��ͼ2��װ�ã���˵��������Ȫ�ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³�����1��03�µ�Ԫ���� ���ͣ�ѡ����
����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�顣

��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2���ռ�����Ӧʹ�� ����Ҫ�õ�����İ�����ѡ�� ���������
��3����ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ����� ����ʵ���ԭ���� ��
��4�����ֻ�ṩ��ͼ2��װ�ã���˵��������Ȫ�ķ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com