
����Ŀ������֪��Ӧ��R��CH��CH��O��R��(����ϩ����) ![]() R��CH2CHO + R��OH
R��CH2CHO + R��OH
����ϩ����A����Է�������Mr(A)=162��ˮ�����B����Է�������Mr(B)=46����A��صĻ�ѧ��Ӧ���£���ش��������⣺
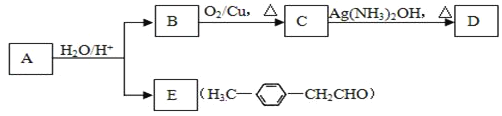
(1)A�Ľṹ��ʽΪ______________________���÷�������_________�ֻ�������ԭ�ӡ�
(2)B��������__________��д��B��C��Ӧ�Ļ�ѧ����ʽ��________________________��
(3)д��C �� D��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
(4)д��ͬʱ��������������E������ͬ���칹��Ľṹ��ʽ�������ڷ���ȩ �ڱ����������ֲ�ͬ��������ԭ��_________________________��
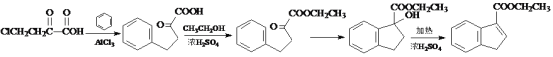
II����֪�� ![]() +ClCH2CH3
+ClCH2CH3 +HCl
+HCl
(5)��д����![]() ��B�ͱ���Ϊԭ�ϣ��ϳ�
��B�ͱ���Ϊԭ�ϣ��ϳ�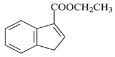 ������ͼ(ע�����Լ�����)____________________��
������ͼ(ע�����Լ�����)____________________��![]()
![]()
���𰸡�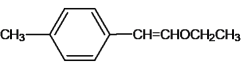 7 �Ҵ� 2CH3CH2OH+O2
7 �Ҵ� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CHO+2[Ag(NH3)2]OH
2CH3CHO+2H2O CH3CHO+2[Ag(NH3)2]OH![]() CH3COONH4+2Ag+3NH3��+ H2O
CH3COONH4+2Ag+3NH3��+ H2O 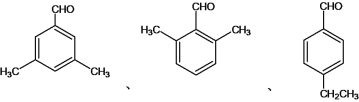
��������
AΪ����ϩ���ѣ�������Ϣ�����Ի����£�����ȩ�ʹ���EΪȩ��BΪ��������B����������ȩ��ȩ�����������ᣬBΪ���������С�CH2OH�Ľṹ����������Է���������BΪ�Ҵ���
(1) AΪ����ϩ���ѣ�������Ϣ�����Ի����£�����ȩ�ʹ���EΪ�Լ�����ȩ��BΪ�Ҵ���������֪��Ϣ��A�Ľṹ��ʽΪ ���÷����ϵĵ�Ч����ͼ����ʾ��
���÷����ϵĵ�Ч����ͼ����ʾ��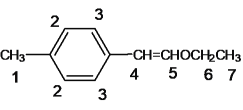 ����7�֣�
����7�֣�
(2) AΪ����ϩ���ѣ�������Ϣ�����Ի����£�����ȩ�ʹ��� BΪ��������B����������ȩ��ȩ�����������ᣬBΪ���������С�CH2OH�Ľṹ����������Է�������Ϊ46��BΪ�Ҵ���B����C�ķ�ӦΪ���Ĵ���������ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(3)CΪ��ȩ����������Һ�õ�����泥���ѧ����ʽΪCH3CHO+2[Ag(NH3)2]OH![]() CH3COONH4+2Ag+3NH3��+ H2O��
CH3COONH4+2Ag+3NH3��+ H2O��
(4)�����ڷ���ȩ������������CHO������������2�ֲ�ͬ��������ԭ�ӣ���һ���������ڶ�λ������2����CH3���ڶԳ�λ�á��� ��
��
(5) ![]() ��B�Ҵ����Է���������Ӧ�õ������е�������������֪��
��B�Ҵ����Է���������Ӧ�õ������е�������������֪��![]() �뱽����ȡ����Ӧ���õ�������̼̼˫�����ô�����ȥ��Ӧ�õ�������ͼΪ��
�뱽����ȡ����Ӧ���õ�������̼̼˫�����ô�����ȥ��Ӧ�õ�������ͼΪ�� ��
��


 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʽṹ��ʾ���ʹ��ɵİ��أ���ش��������⣺
��1��Ԫ��A����8�����ӣ�10�����ӵĺ��أ��ú��ط��ű�ʾΪ______��Ԫ��B����ѧ�ҳ�������֮��������Ԫ��Aͬ����λ�ڵ������ڣ�Ԫ��B������Ϊ______��A��B���⻯��е�ϸߵ���_____���ѧʽ����
��2��CH4�й��õ��Ӷ�ƫ��C��SiH4�й�Ԫ��Ϊ+4�ۣ���C��Si��H�ĵ縺���ɴ�С��˳��Ϊ______��
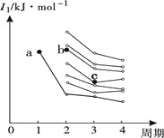
��3����ͼ��ǰ����������Ԫ�ص�һ�������ݶ�ͼ��ͼ��a���Ӧ��Ԫ��Ϊ�⣬��b���ӦԪ�ص�δ�ɶԵ�����Ϊ______��c���ӦԪ�ػ�̬ԭ�Ӽ۵����Ų�ʽΪ_______��
��4��FeC13����������ˮ���Ҵ����þƾ��Ƽ��ȼ�����������FeF3�����۵����1000�棬�Խ������ֻ������۵����ϴ��ԭ����____��
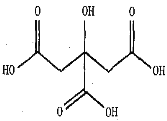
��5��ˮ���к��в�ͬ�Ĺ��ᣬ�������ʡ����ٵ�ˮ���к��������ᣬ������Ľṹ����ͼ��������ľ�������Ϊ_____��̼ԭ�ӵ��ӻ��������Ϊ_____�������ᾧ���к��е���������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£�ij�����м���������̼��ƣ�������ӦCaCO3(s)![]() CaO(s)+CO2(g)���ﵽƽ�⣬����˵����ȷ����
CaO(s)+CO2(g)���ﵽƽ�⣬����˵����ȷ����
A. �������СΪԭ����һ�룬����ϵ�ٴδﵽƽ��ʱ��CO2��Ũ�Ȳ���
B. ���������Ϊԭ����2�����ٴδﵽƽ��ʱ��������ܶȱ��
C. ��CaCO3(s)����������ŷֽ�����CaO(s)��CO2(g)����������H<0
D. ��������ѹǿ���䣬����He��ƽ�����淴Ӧ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪:DΪ��;E������̼Ԫ������Ԫ�ص�����֮��6��1,��Է�������Ϊ44,��ȼ�ղ���ֻ��CO2��H2O��A�����ʽ��F��ͬ,���ܷ���������Ӧ,���ɵ���ˮ��õ���
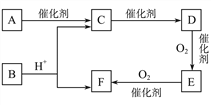
��1��A�Ľṹ��ʽΪ__________________��
��2��д��D��E�Ļ�ѧ����ʽ:_______________________��
��3������˵����ȷ����____��
A.�л���F��ʹʯ����Һ���
B.�����Ƶ�������ͭ�������л���C��E��F��ˮ��Һ
C.�����ʵ�����C��D�ֱ���ȫȼ�����������������
D.���ñ���̼������Һ��ȥ�л���B�л��е�����C��F
E. B��ͬ���칹�����ܷ���������Ӧ�������2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�������衢��������Ͷ�����̼���������������ѧ���ʾ���һ���������ԣ�þ���ƵĻ�ѧ����Ҳ����һ���������ԡ�

������ͼ��ʾװ�ý���þ�Ͷ��������ʵ�飬����A���Ʊ���������ķ���װ�á�
��1��ѡ����ȡ��������ĺ����Լ�________(�����)��
��10%��������Һ����80%������Һ�����������ƹ��塡��������ƹ���
��2��д��װ��B�з�����Ӧ�Ļ�ѧ����ʽ�� ______________________��
��3������Ϊ��װ�õIJ���֮����_______________________________(��д2��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�ʾʽ�������(����)
A. Na���Ĺ����ʾʽ��![]() B. Na���Ľṹʾ��ͼ��
B. Na���Ľṹʾ��ͼ��![]()
C. Na�ĵ����Ų�ʽ��1s22s22p63s1 D. Na����Χ�����Ų�ʽ��3s1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ�Ǻϳ������ᡢ����ͪ�����ӵȵ���Ҫԭ�ϣ�Ҳ������ʯ����ȡ����������ֵ�����ȶ�����
�Ʊ�����ϩ�ķ�Ӧԭ��������Ӧ![]() ������Ӧ
������Ӧ![]()
�Ʊ�����ϩ��ʵ��װ��ͼ���£�(�г�װ�ü�����װ����ʡ��)
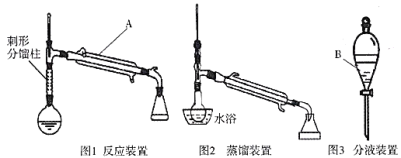
��Ӧ��ʵ����������ͼ��ʾ��

������ʷе㡢�ܶȡ��ܽ������£�
�е�/�� | �ܶ�/( | ˮ���ܽ��� | |
������ | 161 | 0.962 4 | ������ˮ |
����ϩ | 83 | 0.811 | ������ˮ |
85% | 1.69 | ������ˮ | |
����ϩ��ˮ�γɵĹ�����(��ˮ10%) | 70.8 | ||
��������ˮ�γɵĹ�����(��ˮ80%) | 97.8 |
�ش��������⣺
(1)ʵ���в���Ũ���ᣬ����85%![]() ��Һ��˵������________________(д��һ������)��
��Һ��˵������________________(д��һ������)��
(2)��ֲ��ﻷ��ϩ�м���ʳ��ʹˮ�㱥�͵�Ŀ����_____________��ˮԡ����ǰ������ˮ�Ȼ��Ƶ�Ŀ����___________________________.
(3)����A��B�����Ʒֱ���________________________��
(4)�����ᴿʱ�õ�ˮԡ���ȵķ��������ŵ���______________(д����������)��
(5)��������Ҫ��ʵ��ķ�Ӧ�¶�Ӧ������90������ԭ����_____________________��
(6)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ2SO2+O2![]() 2SO3���ܱ������н��С�����˵����ȷ���ǣ� ��
2SO3���ܱ������н��С�����˵����ȷ���ǣ� ��
A.ʹ�ô������ܸı䷴Ӧ����
B.��ƽ��ʱSO2��SO3��Ũ�����
C.����ѹǿ�ܼӿ췴Ӧ����
D.SO2��O2��Ӧ����ȫת��ΪSO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵�����ʾ������ȷ����
A.1mol��������2mol��������ȫȼ��ʱ��ȼ������ͬ
B.��֪��2H2(g)��O2(g)=2H2O(l) ��H=��571.6kJ��mol��1����H2��ȼ����Ϊ285.8kJ��mol��1
C.��ϡ��Һ�У�H��(aq)��OH��(aq)=H2O(aq)��H=��57.3kJ��mol��1��������1molCH3COOH�Ĵ�����Һ�뺬1molBa(OH)2����Һ��ϣ��ų�������С��57.3kJ
D.��101kPa��25��ʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ2H2(g)��O2(g)=2H2O(l) ��H=��285.8kJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com