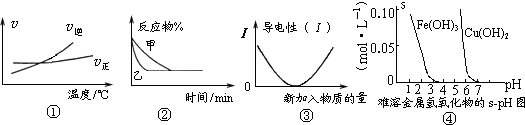
����⣺��1���к���������Aֻ��NaOH��NH
3?H
2O�����ʵ�����A������ʵ���Ũ��B��D��ϳ����ԣ�D�������٣�˵��D���Ա�B�ļ���ǿ������D��NaOH���ʴ�Ϊ��NaOH��
��2���١���NH
3?H
2O?OH
-+NH
4+��֪����ˮ�ٽ����룬��n��NH
3?H
2O�����٣�n��OH
-��������
=
��С���ʢٴ���
�ڡ���NH
3?H
2O?OH
-+NH
4+��֪����ˮ�ٽ����룬��n��NH
3?H
2O�����٣�n��OH
-������c��NH
3?H
2O����c��OH
-��������c��H
+��������
��С���ʢ���ȷ��
�ۡ����ˮϡ��ʱ���¶Ȳ��䣬��c��H
+����c��OH
-���ij˻����䣬�ʢ۴���
�ܡ���NH
3?H
2O?OH
-+NH
4+��֪����ˮ�ٽ����룬OH
-�����ʵ������ʢܴ���
�ʴ�Ϊ���٢ڣ�
��3��������������ʵ���Ũ��NH
3?H
2O����NH
4HSO
4��Ϻ���Һ������Ӧ��NH
3?H
2O+NH
4HSO
4=��NH
4��
2SO
4+H
2O��NH
4��
2SO
4Ҫ����ˮ�⣺NH
4++H
2O?NH
3?H
2O+H
+����Һ�����ԣ�����ˮ��ƽ�������ƶ���c��H
+������pH��С���ʴ�Ϊ���ܣ�
��4���ٸ���������п��Ӧ�õ����������ŷ�Ӧ�Ľ��У�CH
3COOH���ϵ����H
+����Ӧ���ʱ������п죬����������һ���࣬��Ӧ����Ҫ��ʱ��HCl��CH
3COOH���ʢٴ���
�ڸ���������п��Ӧ�õ���������ʼ��Һ��c��H
+����ȣ���Ӧʱ������HCl=CH
3COOH���ʢڴ���
�۸���������п��Ӧ�õ���������������һ����Һ�д���п���ų�������������ͬ��˵��������пʣ�࣬CH
3COOH��п��ȫ��Ӧ���μӷ�Ӧ��п�����ʵ�����ȣ��ʢ���ȷ��
�ܸ���������п��Ӧ�õ����������ŷ�Ӧ�Ľ��У�CH
3COOH���ϵ����H
+����Ӧ���ʱ������п죬�ʢ���ȷ��
�ݸ���������п��Ӧ�õ���������������һ����Һ�д���п���ų�������������ͬ��˵��������пʣ�࣬CH
3COOH��п��ȫ��Ӧ���μӷ�Ӧ��п�����ʵ�����ȣ��ʢ���ȷ��
����������п��Ӧ�õ���������������һ����Һ�д���п���ų�������������ͬ��˵��������пʣ�࣬CH
3COOH��п��ȫ��Ӧ���μӷ�Ӧ��п�����ʵ�����ȣ��ʢ���
�ʴ�Ϊ���ۢܢݣ�
��5��NH
4HSO4�е���NaOH��Һ��NaOH������NH
4HSO
4�������H
+���ã���ΪH
+���OH
-��������NH
4+���OH
-������ǿ��ԭ���Dz���H
2O��NH
3?H
2O���ѵ��룩���������Ħ����NaOHʱ�����ý�H
+�кͣ���ʱc��Na
+��=c��SO
42-��������ʱ��Һ�л���NH4+��NH
4+ˮ��ʹ��Һ�����ԣ����Ҫʹ��Һ�����ԣ������������NaOH����Ȼ������ʱc��OH
-��=c��H
+����c��Na
+����c��SO
42- ����c��NH
4+�����ʴ�Ϊ��c��Na
+����c��SO
42-����c��NH
4+����c��OH
-��=c��H
+����








 H++Cl-+HClO�������CaCO3��ĩ��H+��Ӧ��ƽ�������ƶ���HClOŨ������
H++Cl-+HClO�������CaCO3��ĩ��H+��Ӧ��ƽ�������ƶ���HClOŨ������ H++Cl-+HClO�������CaCO3��ĩ��H+��Ӧ��ƽ�������ƶ���HClOŨ������
H++Cl-+HClO�������CaCO3��ĩ��H+��Ӧ��ƽ�������ƶ���HClOŨ������