
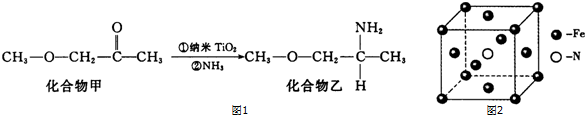
���� ��1����ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d5�����Ӳ�ͬ�˶�״̬��ͬ��
��2��NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
��3��N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�N4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��ÿ����Ϊ�������Σ�N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ��
��4��NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�
��5������Ĵ��ڵ��������۷е����ߣ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��6�����Ͱ�����640��ɷ����û���Ӧ���������͵����������þ�̯��ȷ���������Ļ�ѧʽ�������¶ȡ���Ӧ���������д����Ӧ����ʽ��
�ɾ������V=$\frac{m}{��}$����������̶���������������ⳤ��������������ⳤ���������Feԭ�Ӽ�ľ��룮
��� �⣺��1����ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d6��[Ar]3d6��TiԪ��ԭ�Ӻ�����22�����ӣ�����ԭ�����˶�״̬��ͬ�ĵ��ӹ���22�֣�
�ʴ�Ϊ��1S22S22P63S23P63d6��[Ar]3d6��22��
��2��NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�NH3��Nԭ�Ӻ���3���Ҽ���1���µ��Ӷԣ�����NH3Ϊ�������ͣ�BF3��Bԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�BF3•NH3�����к��еĻ�ѧ�������й��ۼ�����λ������BF3•NH3��Bԭ�ӵ��Ӷ���Ϊ$\frac{5+3}{2}=4$�����ӻ���ʽΪsp3��
�ʴ�Ϊ��ƽ���������Σ������ͣ����ۼ�����λ����sp3��
��3��N4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���ÿ����Ϊ�������Σ�N-N ���ļ���Ϊ60�㣬N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ��
�ʴ�Ϊ��60�㣻�������ƽ�����ըҩ��
��4��F�ĵ縺�Դ���NԪ�أ�NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�����NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ��Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�����NF3������Cu2+�γ������ӣ�
�ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��F������NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ����
��5������Ĵ��ڵ��������۷е����ߣ����к���������ײ�����������Ի��������۷е���ڼף����γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������N��O��C��
�ʴ�Ϊ���������ҷ��Ӽ���������N��O��C��
��6���þ�������ԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ԭ�Ӹ�����1�����Ե������Ļ�ѧʽ��Fe4N�����Ͱ�����640��ɷ����û���Ӧ���������͵����������Ը÷�Ӧ����ʽΪ��8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2��һ��������Feԭ����ĿΪ$8��\frac{1}{8}+6��\frac{1}{2}=4$��Nԭ����ĿΪ1�������ⳤa=$\root{3}{\frac{m}{��}}$=$\root{3}{\frac{56��4+14}{��{N}_{A}}}$�������������Feԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}a$
=$\frac{\sqrt{2}}{2}\root{3}{\frac{238}{��{N}_{A}}}$��
�ʴ�Ϊ��8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2��$\frac{\sqrt{2}}{2}\root{3}{\frac{238}{��{N}_{A}}}$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰���ӽṹ���ӻ�������縺�ԡ���������Ų�ʽ�������ṹ�ȣ�ע��������λ�����йظ���Ѷ��еȣ�


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
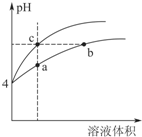 ���ᡢ�����̼���ǻ�ѧʵ����о��г��õļ����ᣮ�����£�Ka��CH3COOH��=1.7��10-5 mol/L��H2CO3�ĵ��볣��Ka1=4.2��10-7mol•L-1��Ka2=5.6��10-11mol•L-1
���ᡢ�����̼���ǻ�ѧʵ����о��г��õļ����ᣮ�����£�Ka��CH3COOH��=1.7��10-5 mol/L��H2CO3�ĵ��볣��Ka1=4.2��10-7mol•L-1��Ka2=5.6��10-11mol•L-1| ʵ���� | ����������Һ��Ũ�� ��mol•L-1�� | �ζ����ʱ������������Һ����������mL�� | ��������������mL�� |
| 1 | 0.10 | 24.12 | 20.00 |
| 2 | 0.10 | 23.88 | 20.00 |
| 3 | 0.10 | 24.00 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��Na+����c��F-�� | B�� | c��Na+����c��F-�� | ||
| C�� | c��Na+��=c��F-�� | D�� | ��ȷ��c��Na+����c��F-���Ĵ�С��ϵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�鷽�� | ʵ�鷽�� | �������� | |
| A | �ζ��� | ����Ʒ���100mL��Һ��ȡ10.00mL�����˼��ȣ��ñ�����ζ� | ������������ |
| B | ������ | ����Ʒ�����ᷴӦ��ʹ���ɵ�����ȫ������ʯ������ | ��ʯ������ |
| C | ������ | ����Ʒ�����ձ��У�������ƽ�ϣ������������� | ��������� |
| D | ������ | ����Ʒ�����ᷴӦ������ͨ����ˮ����װ������ | ��ˮ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AlCl3��aq�� | B�� | Al2��SO4��3��aq�� | C�� | HCl��aq�� | D�� | NaHCO3��aq�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
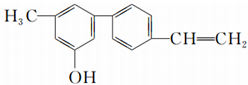
| A�� | ���л�������ϩ�� | B�� | ���л������ڴ� | ||
| C�� | ���л�����������е�ԭ�Ӷ����� | D�� | ���л��������ֹ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 431kJ | B�� | 945.6kJ | C�� | 649kJ | D�� | 869kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HX | B�� | H2X | C�� | XH3 | D�� | XH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ��Ũ����
��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ��Ũ����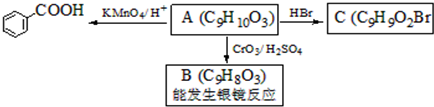
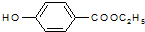
 ������ȡ����Ӧ���Ӧ���ͣ���
������ȡ����Ӧ���Ӧ���ͣ��� ��
�� ��
�� ����һ��ҽҩ�м��壬����ƺ���������
����һ��ҽҩ�м��壬����ƺ���������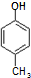 �ϳ�D��������ԭ����ѡ���÷�Ӧ����ͼ��ʾ��ע����Ҫ�ķ�Ӧ��������
�ϳ�D��������ԭ����ѡ���÷�Ӧ����ͼ��ʾ��ע����Ҫ�ķ�Ӧ��������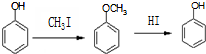
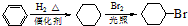
 ��
���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com