
ij��Һ�п��ܺ�������5�������е�ij���֣�Na+��NH4+��Mg2+��Al3+��Cl����Ϊȷ�ϸ���Һ��ɽ�������ʵ�飺��ȡ20.0 mL����Һ������25.0 mL 4.00 mol��L-1NaOH��Һ���а�ɫ���������ݼ���ζ���塣���ˡ�ϴ�ӡ�����ó���1.16 g���ٽ���Һϡ����100 mL�������Һ��c(OH��)Ϊ0.20 mol��L-1������ȡ20.0 mL����Һ������������AgNO3��Һ�����ɰ�ɫ����11.48 g���ɴ˿ɵó�����ԭ��Һ��ɵ���ȷ������
A��һ������Mg2+��Al3+��Cl��������Na+��NH4+
B��һ������Na+��Mg2+��Cl��������NH4+�����ܺ���Al3+
C��c (Cl��) Ϊ 4.00 mol��L-1��c (Al3+) Ϊ1.00 mol��L-1
D��c (Mg2+) Ϊ 1.00 mol��L-1��c(Na+ ) Ϊ 0.50 mol��L-1
D
��������
�������������ʵ����жϣ�ԭ��Һ��һ����Mg2+����NH4+��
�μӷ�Ӧ��OH����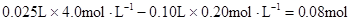
n(Mg2+)= Mg2+ + 2OH��=
Mg(OH)2��
Mg2+ + 2OH��=
Mg(OH)2��
��Mg2+��Ӧ��OH����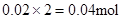 ������ԭ��Һ��һ������Al3+��
������ԭ��Һ��һ������Al3+��
��Al3+��Ӧ��OH����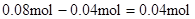
����Һ�л���OH����������Ӧ Al3+ + 4OH��= AlO2�� + 2H2O
n(Al3+ )= 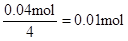
��ʵ��ڽ������ļ������ݣ��� n(Cl��)= 
n(Cl��)��2n(Mg2+) + 3n(Al3+ ) ����ԭ��Һ�л����� Na+
2n(Mg2+) + 3n(Al3+ )+ n(Na+
) = 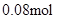 n(Na+ ) =
n(Na+ ) = 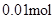
ԭ��Һ�����ӵ�Ũ�ȣ�
c (Cl��) = 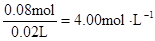 c (Al3+)=
c (Al3+)=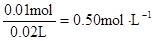
c (Mg2+)=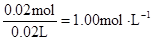 c(Na+ )=
c(Na+ )= 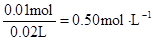
��ѡD��
���㣺���������ӵļ��� ���������ӵļ��� ����Ũ�ȵļ���
���������⿼��ѧ���������ӵļ��鷽���������Ѷȵļ��㣬���Ը�����ѧ��֪ʶ���ش��Ѷ��С�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ������Mg2+��Al3+��Cl��������Na+��NH4+ | B��һ������Na+��Mg2+��Cl��������NH4+�����ܺ���Al3+ | C��c ��Cl���� Ϊ 4.00 mol?L-1��c ��Al3+�� Ϊ1.00 mol?L-1 | D��c ��Mg2+�� Ϊ 1.00 mol?L-1��c��Na+�� Ϊ 1.50 mol?L-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com