
����Ŀ������ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���һ����̼�������Ļ����������ú̿��ˮ(����)��Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ___________________��
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ��C3H8(g)+5O2(g)��3CO2(g)+4H2O(l) ��H=��2 220.0 kJmol1����֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��CO(g)+1/2O2(g)��CO2(g) ��H=��283.0 kJmol1���ԱȽ���ͬ���ʵ�����C3H8��COȼ�գ�������������ֵԼΪ_________��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ��2H2(g)+O2(g)��2H2O(l) ��H=��571��6 kJmol1���ԱȽ�ͬ�����������ͱ���ȼ�գ�������������ֵԼΪ______��
��4��������δ������Դ����������������֮�⣬�����е��ŵ���___________________��
���𰸡�C+H2O(g)![]() CO+H22220��2831571.9��555��Դ�ḻ����������Ⱦ��
CO+H22220��2831571.9��555��Դ�ḻ����������Ⱦ��
��������
��1��ú̿��ˮ�����ڸ����·�Ӧ����һ����̼��������
��2�������Ȼ�ѧ����ʽ������ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ����������֮�ȣ�
��3�������Ȼ�ѧ����ʽ���㣻
��4������������ȼ�յIJ��P��Դ�����ش�
��1��ú̿��ˮ�����ڸ����·�Ӧ����һ����̼����������Ӧ����ʽΪC+H2O(g)![]() CO+H2��
CO+H2��
��2�������Ȼ�ѧ����ʽ��֪����ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ����������֮��Ϊ2220��283��
��3�������Ȼ�ѧ����ʽ��֪����ͬ�����������ͱ���ȼ�գ�������������ֵΪ![]() ��
��
��4���������п�ȼ�ԣ�ȼ�յIJ�����ˮ����Ⱦ��������ˮ��ȡ��������Դ�㡣


 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д� ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������ȵĹ̶��ݻ����ܱ������У��������淴ӦmA(g) + nB(g) ![]() pC(g) + qD(g)����m��n��p��qΪ��������ʱ����Ӧ�ﵽƽ��ı�־��( )
pC(g) + qD(g)����m��n��p��qΪ��������ʱ����Ӧ�ﵽƽ��ı�־��( )
����ϵ�¶Ȳ��ٸı�
�۸���ֵ�Ũ�Ȳ��ٸı�
�ܸ���ֵ������������ٸı�
�ݷ�Ӧ����v(A)��v(B)��v(C)��v(D)=m��n��p��q
��λʱ����m mol A�ϼ���Ӧ��ͬʱp mol CҲ�ϼ���Ӧ
����ϵ���ܶȲ��ٱ仯
A���ۢܢݢ� B���ڢۢܢ�
C���٢ۢܢ� D���ۢܢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ��
��1��1 mol C2H4(g)������O2(g)��Ӧ����CO2(g)��H2O(l)���ų�1411 kJ������ ��
��2��1 mol C2H5OH(l)������O2(g)��Ӧ����CO2(g)��H2O(l)���ų�1366.8 kJ������ ��
��3��2 mol Al(s)������O2(g)������Ӧ����Al2O3(s)���ų�1669.8 kJ������ ��
��4����200��C��101 kPaʱ��1 mol H2���������������HI���壬�ų�14.9 kJ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺

��1����1���������к��Ȳⶨʵ�飬�¶ȼ�������ʹ��________�Σ�ijͬѧΪ��ʡȥ��ϴ�¶ȼƵ��鷳������ʵ��ʱʹ����֧�¶ȼƷֱ������ͼ���¶ȣ����Ƿ�ͬ���ͬѧ�Ĺ۵㣬Ϊʲô��_______________��
��2��ʵ������50 mL 0.50 mol��L��1���ᣬ50 mL 0.55 mol��L��1 NaOH��Һ���вⶨ�к��ȵ�ʵ�飬Ϊ�˰Ѳ������ݼ�¼������������Ʊ���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W����������һ�������¾�������ͼ��ʾ��ת����ϵ�������жϴ������

A. ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����Z������NaAlO2
B. ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ�ǽ�������ʱ����Z������CO2
C. ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ��X������NH3
D. ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ��������ʱ����Z������FeCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Է���������̼������ʵ����ɫ�������������ã����ڼ�������Ҳ������Ҫ���塣
��1������β������Ҫ��Ⱦ��ΪNO����H2����ԭNO���Դﵽ������Ⱦ��Ŀ�ġ�
��֪��2NO(g) ![]() N2(g)��O2(g) ��H=��180.5 kJ��mol��1
N2(g)��O2(g) ��H=��180.5 kJ��mol��1
2H2O(l)===2H2(g)��O2(g) ��H=��571.6 kJ��mol��1
д��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ��______________��
��2��ij�о�С��ģ���о����£���2 L�����ܱ������г���2 mol NO������Ӧ2NO(g) ![]() N2(g)��O2(g)���ڲ�ͬ���¶��£���Ӧ���������ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ��
N2(g)��O2(g)���ڲ�ͬ���¶��£���Ӧ���������ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ��
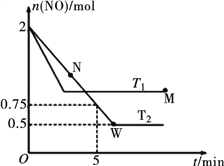
��T2�£���0��5 min�ڣ�v(O2)=______________mol��L��1��min��1�����¶��·�ӦN2(g)��O2(g) ![]() 2NO(g)��ƽ�ⳣ��K=______________��
2NO(g)��ƽ�ⳣ��K=______________��
���÷�Ӧ���е�M��ų�������______________���е�W��ų�������(��������������������=��)��
M��ʱ�ټ���һ����NO��ƽ���NO��ת����______________(���������������С������������)��
����Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯����______________(�����)��
a�����������ܶ� b���淴Ӧ����
c����λʱ���ڣ�N2��NO��������֮�� d�������ƽ����Է�������
��3��������Ϊһ������ȼ�ϣ�����������������䡣����������Ҫѡ����ʵĴ�����ϣ������Ͻ���һ�������¿����������γ��⻯�LaNi5(s)��3H2(g) ![]() LaNi5H6(s) ��H��0����ʹLaNi5H6(s)�ͷų���̬�⣬����ƽ���ƶ�ԭ�����ɸı��������______________(����ĸ���)��
LaNi5H6(s) ��H��0����ʹLaNi5H6(s)�ͷų���̬�⣬����ƽ���ƶ�ԭ�����ɸı��������______________(����ĸ���)��
A������LaNi5H6(s)���� B�������¶�
C��ʹ�ô��� D����Сѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ȳ�뱽������ȫȼ�յ��Ȼ�ѧ����ʽ���£�
��C2H2(g)��![]() O2(g)�D��2CO2(g)��H2O(l) ��H����1 300 kJ��mol��1
O2(g)�D��2CO2(g)��H2O(l) ��H����1 300 kJ��mol��1
��C6H6(g)��![]() O2(g)�D��6CO2(g)��3H2O(l) ��H����3 295 kJ��mol��1
O2(g)�D��6CO2(g)��3H2O(l) ��H����3 295 kJ��mol��1
����˵����ȷ���� (����)��
A. 1 mol C2H2(g)��ȫȼ��������̬ˮʱ���ȴ���1 300 kJ
B. 1 mol C6H6(l)��ȫȼ������Һ̬ˮʱ���ȴ���3 295 kJ
C. ��ͬ�����£���������C2H2(g)��C6H6(g)��ȫȼ�գ�C6H6(g)���ȸ���
D. C2H2(g)��������C6H6(g)�Ĺ������ڷ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��W��X��Y��Z��M��G���ֶ�����Ԫ�أ�ԭ��������������W��Zͬ���壬���γ����ӻ�����ZW��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ�X����̬�⻯��ˮ��Һ�ʼ��ԡ���ش���������:
��1��Y��Ԫ�����ڱ��е�λ��Ϊ___________��
��2�� W��Y��Z��G�γɵļ����ӵİ뾶��С˳����___________(�û�ѧ���ű�ʾ)
��3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������________ (��д����)
��4��ZW�ĵ���ʽΪ___________��W2Y2�ĵ���ʽΪ______
��5��MY2��G2����ʹƷ����Һ��ɫ�����³�ѹ��������ͬ�����MY2��G2����ͬʱͨ��Ʒ����Һ������������ӷ���ʽ����ԭ��________��
��6����֪
������ | MgO | Al2O3 | MgCl2 | AlCl3 |
���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
�۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ������___________ ������ʱ�����Al2O3�������AlCl3��ԭ����___________ ��
��7����������������ѧ��FulvioCacace���˻���˼��������о������N4���ӡ�N4���ӽṹ����ͼ��ʾ����֪����1 mol N��N����167 kJ����������1 mol N��N�ų�942kJ����������������Ϣ�����ݣ�����˵����ȷ����___________ ��

A��N4����һ�����ͻ����� B��N4�����۵�ߣ�Ӳ�ȴ�
C����ͬ������N4����������N2 D��1molN4ת��ΪN2������882kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.��Ԫ�ص�ͬ���������������ͬ
B.H2O2������������ֻ��������
C.��CaCl2��Һ��ͨ��CO2���壬�а�ɫ��������
D.����β���е�NO��Ҫ�ǵ����������������������γɵ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com