
(11��)��1��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CO2��3mol H2��һ�������·�����Ӧ��
CO2(g)��3H2(g)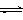 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬ƽ����Ӧ����v(CO2)�� mol/(L��min)��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ________________________________________��
�����д�ʩ����ʹn(CH3OH)��n(CO2)�������������������������������
| A�������¶� | B������He(g)��ʹ��ϵѹǿ���� |
| C����H2O(g)����ϵ�з��� | D���ٳ���1mol H2 |
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ�����������������������������������������������������������������  �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��11 �֣���ѧ��һֱ�����о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����Ӧ�Ļ�ѧ����ʽ���£�N2(g)+ 3H2O(l) 2NH3(g)+ O2(g)���ش��������⣺
��1����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±������ա�N2ѹ��1.0��105Pa����Ӧʱ��3 h������÷�Ӧ������ӦΪ ��Ӧ������ȡ����ȡ���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6 mol�� | 4.8 | 5.9 | 6.0 |
��2����Ŀǰ�㷺ʹ�õĹ�ҵ�ϳɰ�������ȣ��÷����й̵���Ӧ�������������������䷴Ӧ����������NH3�������Ľ��飺���� ��
��3���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá������ڸ�������ˮ������Ӧ��Ӧ����ʽΪ��CH4(g)��H2O(g)��CO(g)��3H2(g)���������ʵ�ȼ������
�����£�
H2(g) ����H=��285.8 kJ・mol��1��
CO(g) ����H =��283.0 kJ・mol��1��
CH4(g) ����H=��890.3 kJ・mol��1 ��
��֪1mol H2O(g)ת��Ϊ1mol H2O(l)ʱ�ų�44.0 kJ������д��CH4��H2O�ڸ����·�Ӧ���Ȼ�ѧ����ʽ__________________________________��
��4����������Ѱ���ʺϵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl����NH4ClΪ�������Һ�Ƴ�����ȼ�ϵ�أ���д���õ缫��������Ӧʽ
��5�����ɵ�NH3���������̬���ʣ���(NH4)2SO4��NH4Cl����Щ������ �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��___________________________��ʹ��ʱ������________________���ʺ�ʩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��) ��1��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CO2��3mol H2��һ�������·�����Ӧ��
CO2(g)��3H2(g)CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬ƽ����Ӧ����v(CO2)�� mol/(L��min)��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ________________________________________��
�����д�ʩ����ʹn(CH3OH)��n(CO2)�������������������������������
A�������¶� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з��� D���ٳ���1mol H2
��2�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2��2CO��O2����CO������ȼ�ϡ�
��֪�÷�Ӧ��������ӦΪ��4OH�D�D4e�D��O2����2H2O
��������ӦʽΪ�� ��
���������������Ʒ�Ӧ2CO��2C��O2����H��0����S��0��������CO����Ⱦ�������ж��Ƿ���в�˵�����ɣ����������������� ������������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��11 �֣���ѧ��һֱ�����о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����Ӧ�Ļ�ѧ����ʽ���£�N2(g)+ 3H2O(l)  2NH3(g)+ O2(g)���ش��������⣺
2NH3(g)+ O2(g)���ش��������⣺
��1����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±������ա�N2ѹ��1.0��105 Pa����Ӧʱ��3 h������÷�Ӧ������ӦΪ ��Ӧ������ȡ����ȡ���
|
T/K |
303 |
313 |
323 |
|
NH3������/��10-6 mol�� |
4.8 |
5.9 |
6.0 |
��2����Ŀǰ�㷺ʹ�õĹ�ҵ�ϳɰ�������ȣ��÷����й̵���Ӧ�������������������䷴Ӧ����������NH3�������Ľ��飺���� ��
��3���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá������ڸ�������ˮ������Ӧ��Ӧ����ʽΪ��CH4(g)��H2O(g)��CO(g)��3H2(g)���������ʵ�ȼ������
�����£�
H2(g) ����H =��285.8 kJ・mol��1��
CO(g) �� ��H =��283.0 kJ・mol��1��
CH4(g) ����H =��890.3 kJ・mol��1 ��
��֪1mol H2O(g)ת��Ϊ1mol H2O(l)ʱ�ų�44.0 kJ������д��CH4��H2O�ڸ����·�Ӧ���Ȼ�ѧ����ʽ__________________________________��
��4����������Ѱ���ʺϵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl����NH4ClΪ�������Һ�Ƴ�����ȼ�ϵ�أ���д���õ缫��������Ӧʽ
��5�����ɵ�NH3���������̬���ʣ���(NH4)2SO4��NH4Cl����Щ������ �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��___________________________��ʹ��ʱ������________________���ʺ�ʩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ��ݸ�и߶��ڶ�ѧ����ĩ���Ի�ѧB�� ���ͣ�ʵ����
(11��) ��1��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CO2��3mol H2��һ�������·�����Ӧ��
CO2(g)��3H2(g) CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬ƽ����Ӧ����v(CO2)�� mol/(L��min)��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ________________________________________��
�����д�ʩ����ʹn(CH3OH)��n(CO2)�������������������������������
A�������¶� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з��� D���ٳ���1mol H2
��2�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2��2CO��O2����CO������ȼ�ϡ�
��֪�÷�Ӧ��������ӦΪ��4OH�D�D4e�D��O2����2H2O
��������ӦʽΪ�� ��
���������������Ʒ�Ӧ2CO��2C��O2����H��0����S��0��������CO����Ⱦ�������ж��Ƿ���в�˵�����ɣ����������������� ������������������������������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com