
��10�֣��״���һ�ַdz��õ�ȼ�ϡ��۵�-97.8��C���е�64.5��C��һ�������£�
CO��H2��Ӧ���Ƶü״���CO+2H2 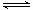 CH3OH
CH3OH
ͼ1��ʾ�÷�Ӧ���й����е������仯��
ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g�� CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
A�������¶� B���Ӵ���
C�����������ʹ��ϵѹǿ���� D���ٳ���1molCO��2molH2
E�����º��ݸ�Ϊ���º�ѹ
��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ ��������ĸ��
A��NaOH���㣬HCOOHʣ��
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 CH3OH
CH3OH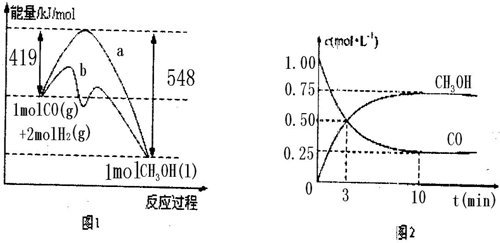
| 3 |
| 2 |
| 3 |
| 2 |
 CH3OH��g����H��0��ƽ�ⳣ��Ϊ
CH3OH��g����H��0��ƽ�ⳣ��Ϊ| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣��״���һ�ַdz��õ�ȼ�ϡ��۵�-97.8��C���е�64.5��C��һ�������£�
CO��H2��Ӧ���Ƶü״���CO+2H2 CH3OH
ͼ1��ʾ�÷�Ӧ���й����е������仯��
ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g�� CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
A�������¶� B���Ӵ���
C�����������ʹ��ϵѹǿ���� D���ٳ���1molCO��2molH2
E�����º��ݸ�Ϊ���º�ѹ
��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺
c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ ��������ĸ��
A��NaOH���㣬HCOOHʣ��
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣��״���һ�ַdz��õ�ȼ�ϡ��۵�-97.8��C���е�64.5��C��һ�������£�
CO��H2��Ӧ���Ƶü״���CO+2H2 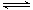 CH3OH
CH3OH
ͼ1��ʾ�÷�Ӧ���й����е������仯��

ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g�� CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ����
�����ı����������������COת���ʵ���
��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ����
�����ı����������������COת���ʵ���
��
A�������¶� B���Ӵ���
C�����������ʹ��ϵѹǿ���� D���ٳ���1molCO��2molH2
E�����º��ݸ�Ϊ���º�ѹ
��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺
c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ ��������ĸ��
A��NaOH���㣬HCOOHʣ��
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�״���һ�ַdz��õ�ȼ�ϡ��۵�-97.8��C���е�64.5��C��һ�������£�
CO��H2��Ӧ���Ƶü״���CO+2H2��������CH3OH
�� ��ͼ1��ʾ�÷�Ӧ���й����е������仯��
�� ��ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
�� ��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ���� ���������������������������� ��
�� ��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g������![]() CH3OH��g����ƽ�ⳣ��Ϊ �� ��10min�������ڵ�ѹǿ��Ϊԭ�������� �����ı����������������COת���ʵ����� ��
CH3OH��g����ƽ�ⳣ��Ϊ �� ��10min�������ڵ�ѹǿ��Ϊԭ�������� �����ı����������������COת���ʵ����� ��
A�������¶ȡ��������������������������������������������������� B���Ӵ�������
C�����������ʹ��ϵѹǿ�������������������� D���ٳ���1molCO��2molH2��������
E�����º��ݸ�Ϊ���º�ѹ
�� ��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺
c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ �������� ��������ĸ��
A��NaOH���㣬HCOOHʣ�ࡡ������������
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com