
| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е㣨�棩 | 56.2 | 77.06 | 78 | 117.9 |
���� �����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����ᣬ
��1�������ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룬�����ᷴӦ���������ƣ�������������Ӧ�����Ҵ��������ƣ�
��2���������Ϸ������Ҵ��ķе�78�������
��3���ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������
��4����������ķе�117.9�������
��� �⣺�����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����
��1�������ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룬�����ᷴӦ���������ƣ�������������Ӧ�����Ҵ��������ƣ���Ӧ����ʽΪ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�ʴ�Ϊ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��2���������Ϸ������Ҵ��ķе�78�棬������70��85��ʱ��������Ҫ�ɷ����Ҵ����ʴ�Ϊ���Ҵ���
��3�������Ϸ������ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������ᣬ����2CH3COONa+H2SO4=Na2SO4+2CH3COOH��
�ʴ�Ϊ����������ת��Ϊ���
��4���������Ϸ���������ķе�117.9�棬��������Ҫ�ɷ������ᣬ�ʴ�Ϊ�����ᣮ
���� ���⿼�����Ʊ���������ƣ���Ŀ�ѶȲ�����ȷ������Ӧԭ��Ϊ���ؼ���ע������������Ӧ��Ũ���ᡢ����̼������Һ��Һ�����ã�����������ѧ���ķ�����������ѧʵ��������


 ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��������һ��һ��10���¿���ѧa���������棩 ���ͣ�ѡ����
������������ȷ����
A��1molO2��������32g��mol-1
B��SO 4 2 - ��Ħ������Ϊ 96 g��mol-1
C��1Ħ���κ����ʵ��������ڸ����ʵ���Է�������
D��CO2��Ħ��������44g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
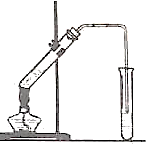 ��ͼ�������Թ����ȼ���2mL95%���Ҵ�������ҡ���»�������3mLŨ���ᣬ�ټ���2mL���ᣬ���ҡ�ȣ������Թ��м���5mL����Na2CO3��Һ����ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮
��ͼ�������Թ����ȼ���2mL95%���Ҵ�������ҡ���»�������3mLŨ���ᣬ�ټ���2mL���ᣬ���ҡ�ȣ������Թ��м���5mL����Na2CO3��Һ����ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
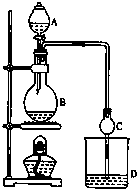 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��| �Լ� | �Ҵ� | ���� | �������� |
| �е�/�� | 78.5 | 118 | 77.1 |
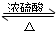 CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g•cm-3�� |
| �Ҵ� | -117.3 | 78.5 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���� | - | 338.0 | 1.84 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�ӦΪ���淴Ӧ�������ܽ��е��ף����Ҵ���ת����һ���ﲻ��100% | |
| B�� | ����ӷ��������������٣��Ҵ����������ܳ��ת��Ϊ�������� | |
| C�� | ���ﲻ�ȶ����ױ�����Ϊ�������ʶ�Ӱ���Ҵ���ת���� | |
| D�� | ����ﵼ�뱥��̼������ҺҺ���ϣ��н϶�����������ܽ���ˮ��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
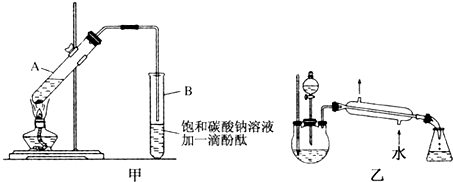
 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ijѧ����ʵ����������ͼ��ʵ��װ���Ʊ������������о��䷴Ӧ������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ijѧ����ʵ����������ͼ��ʵ��װ���Ʊ������������о��䷴Ӧ������| ���� | �е�/��C | �ܶ�/g?cm-3 |
| �Ҵ� | 78.0 | 0.79 |
| ���� | 117.9 | 1.05 |
| �������� | 77.5 | 0.90 |
| ���촼 | 131 | 0.8123 |
| ���������� | 142 | 0.8670 |
| ʵ���� | �Թܢ��е��Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� | 1.2 |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com