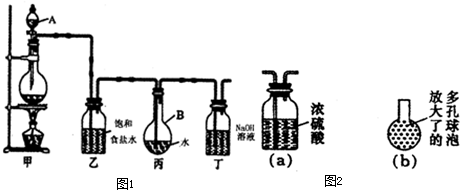
�����仯����������������������й�ϵ
��NO��������Ⱦ����������������������������������NO����������������֯�д��ڣ���������Ѫ�ܡ����ߡ���ǿ����Ĺ��ܣ�����Ϊ��ǰ������ѧ���о����ȵ㣬NO�౻��Ϊ�����Ƿ��ӡ�����ش��������⣮
��1��NO��������
ACD
ACD
�����ţ���
A���ƻ������㡡�� ��B����������ʹһЩ��������
C��������ꡡ���� ��D��������Ѫ�쵰���
��2���ں�Cu
+���ӵ�ø�Ļ�����У�����������ӿ�ת��ΪNO��д��Cu
+������������ӣ�NO
2-��������ˮ��Һ�з�Ӧ�����ӷ���ʽ��
Cu++NO2-+2H+�TCu2++NO��+H2O
Cu++NO2-+2H+�TCu2++NO��+H2O
��
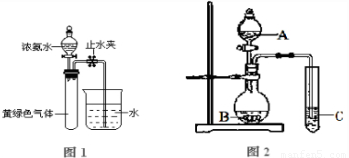
��3����֪NH
3��Cl
2�ᷢ��������ԭ��Ӧ������N
2��NH
4Cl��������ͼ1��ʾ��װ�ý���ʵ�飬��Һ©���Ļ���������Ũ��ˮ�������ٷ�ӦΪֹ���رշ�Һ©���Ļ��������ָ������£���ֹˮ�У��Թ���Һ������������֮������
��д��ʵ������ȡ�����Ļ�ѧ����ʽ
Ca��OH��2+2NH4Cl�TCaCl2+2NH3��+2H2O
Ca��OH��2+2NH4Cl�TCaCl2+2NH3��+2H2O
��
���Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��
8NH3+3Cl2�TN2+6NH4Cl
8NH3+3Cl2�TN2+6NH4Cl
��
���Թ��з����ķ�Ӧ������������
bc
bc
�����ţ���
a�������� b����ԭ�� c������ d���Ȳ��ȶ���
��1����SO
2ͨ����CuSO
4��NaCl��ϵ�Ũ��Һ�У���Һ��ɫ��dz��������ɫ������ȡ�ó�������Ԫ����������������֪���к�Cl��35.7%��Cu��64.3%����SO
2��������Ӧ�е�������
c
c
�����ţ���
a��Ư�� b�������� c����ԭ��
��2����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⣮�ָ���H
2O
2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ
Cu+2H++H2O2�TCu2++2H2O
Cu+2H++H2O2�TCu2++2H2O
��
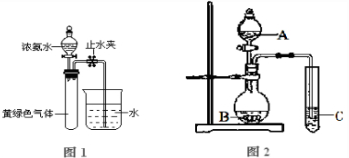
��3������ͼ2ʵ��װ�ÿ���֤����̼��������Ԫ�صķǽ�����ǿ������A��B��C�����������ʵ����ƣ�A
H2SO4
H2SO4
��B
NaHCO3��Na2CO3
NaHCO3��Na2CO3
��C
Na2SiO3
Na2SiO3
�ѧʽ����




 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�