
 +2KMnO4$\stackrel{��}{��}$
+2KMnO4$\stackrel{��}{��}$ +KOH+2MnO2��+H2O
+KOH+2MnO2��+H2O +HCl��
+HCl�� +KCl
+KCl
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У��ܽ� ��ȴ�����ˣ� | �õ���ɫ�������ɫ��Һ | -- |
| �� | ȡ������Һ���Թ��У�����2-3��AgNO3��Һ | ���ɰ�ɫ���� | ��Һ����Cl- |
| �� | �����ɫ���壬����ʹ���ڻ��������۵� | �۵�Ϊ122.4�� | ��ɫ�����DZ����� |
���� һ�����ļױ���������KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ������ͼ���̷����������ͻ���δ��Ӧ�ļױ���������������ˮ���ױ�������ˮ���������ܵ�Һ����÷�Һ�������룬����ʵ��Ŀ��֪���Ӷ��õ��л����ˮ�࣬�л����к��мױ���ˮ���к��б����ᣬ�л����еļױ����������õ���ɫҺ��A��A�Ǽױ�����ˮ�������ữ������Ũ�������ݱ�������ܽ��֪���õ��Ĺ���B�DZ����ᣮ
��1�����뻥�����ܵ�Һ����÷�Һ���������뻥���ҷе㲻ͬ��Һ�����������
��2���ױ���ʹ���Ը��������Һ��ɫ��
��3����Ϸ�Ӧ��ѧ����ʽ�����ɵIJ���������з��벽���������ɫ����B�DZ�������KCl�Ļ������ݱ������۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g���ȼ��鱽����ļ����ټ����Ȼ��صĴ��ڣ�
��4���������KOH��Һ��������кͷ�Ӧ�����ݱ������KOH֮��Ĺ�ϵʽ���㱽������������Ӷ����㱽���������������
��� �⣺һ�����ļױ���������KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ������ͼ���̷����������ͻ���δ��Ӧ�ļױ���������������ˮ���ױ�������ˮ���������ܵ�Һ����÷�Һ�������룬����ʵ��Ŀ��֪���Ӷ��õ��л����ˮ�࣬�л����к��мױ���ˮ���к��б����ᣬ�л����еļױ����������õ���ɫҺ��A��A�Ǽױ�����ˮ�������ữ������Ũ�������ݱ�������ܽ��֪���õ��Ĺ���B�DZ����ᣮ
��1�����뻥�����ܵ�Һ����÷�Һ��������������ͼ�У�ˮ����л�����ܣ����Բ��÷�Һ�������룬������IΪ��Һ���л��������ʻ����ҷе㲻ͬ�����Կ��Բ����������룬������IIΪ����
�ʴ�Ϊ����Һ������
��2��ͨ�����Ϸ���֪��A�Ǽױ����ױ����м��������ܱ����Ը����������Ϊ�������ʹ���Ը��������Һ��ɫ������������Ը��������Һ����ױ���
�ʴ�Ϊ���ױ�������KMnO4��Һ����Һ��ɫ��
��3��ͨ���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ��Ʋ��ɫ����B�DZ�������KCl�Ļ����Ȼ��ؿ����������ữ����������Һ���������ӵĴ��ڣ����ñ�������ܽ��������25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g�����ò�ͬ�¶��µ��ܽ�ȣ���������õ������ͨ���ⶨ�۵��ж��Ƿ�Ϊ�����
�ʴ�Ϊ��
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�м��ȣ��ܽ⣬��ȴ������ | �õ���ɫ�������ɫ��Һ | -- |
| �� | ȡ������Һ���Թ��У�����2-3��AgNO3��Һ | ���ɰ�ɫ���� | ��Һ����Cl- |
| �� | �����ɫ���壬����ʹ���ڻ��������۵㣻 | �۵�Ϊ122.4�� | ��ɫ�����DZ����� |
���� ���⿼�����������ʵ�ʵ������жϣ����ʷ����������Լ�ѡ�������������Ӧ�ã����ʳɷֵ�ʵ����Ʒ��������衢�Լ�����Ʒ���ȵļ��㣬ע�����ʱ�����л�ȡ���õ���Ϣ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | |
| ������ | -89.53 | 117.25 | 0.81 |
| 1-�嶡�� | -112.4 | 101.6 | 1.28 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����[H2NCONH2]����һ�ַdz���Ҫ�ĸߵ����ʣ����Ƕ����л�������Ʒ������ԭ�ϣ���ҵ���Ժϳɰ�����NH3��CO2Ϊԭ���������أ���ش��������⣺
����[H2NCONH2]����һ�ַdz���Ҫ�ĸߵ����ʣ����Ƕ����л�������Ʒ������ԭ�ϣ���ҵ���Ժϳɰ�����NH3��CO2Ϊԭ���������أ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ȩ��+��CH3CO��2O����������$��_{140-170��}^{K_{2}CO_{3}}$
������ȩ��+��CH3CO��2O����������$��_{140-170��}^{K_{2}CO_{3}}$ ������ᣩ+CH3COOH�����ᣩ
������ᣩ+CH3COOH�����ᣩ| ���� | ������ | ��״ | �ܶ�g/cm3 | �۵�� | �е�� | �ܽ�ȣ���/100ml�ܼ� | ||
| ˮ | �� | �� | ||||||
| ����ȩ | 106 | ��ɫҺ�� | 1.06 | -26 | 178-179 | 0.3 | ���� | ���� |
| ������ | 102 | ��ɫҺ�� | 1.082 | -73 | 138-140 | 12 | �� | ���� |
| ����� | 148 | ��ɫ�ᾧ | 1.248 | 133-134 | 300 | 0.04 | 24 | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | ˮ���ܽ�� | |
| ���촼 | 88 | 0.8123 | 131 | �� |
| �� �� | 60 | 1.0492 | 118 | �� |
| ���������� | 130 | 0.8670 | 142 | ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2���� | �²t2-t1���� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����� | ���� | ������ | ����� | ���� | ����� | �ǵ���� |
| ���ڸ�������� | �� | �� | ��� | �� | �ۢݢ� | �٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
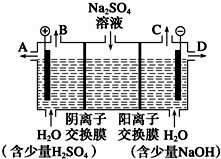 ��Ȼ����â����ѧʽΪNa2SO4•10H2O��Ϊ��ɫ���壬������ˮ����С��ͬѧ���룬���ģ�ҵ�����ӽ���Ĥ�����ռ�ķ���������ͼ��ʾװ�õ����������Һ����ȡ������������������������ƣ����۴ӽ�ʡ��Դ���Ǵ����ԭ�ϵ������ʶ��Զ����ӷ�����ɫ��ѧ���
��Ȼ����â����ѧʽΪNa2SO4•10H2O��Ϊ��ɫ���壬������ˮ����С��ͬѧ���룬���ģ�ҵ�����ӽ���Ĥ�����ռ�ķ���������ͼ��ʾװ�õ����������Һ����ȡ������������������������ƣ����۴ӽ�ʡ��Դ���Ǵ����ԭ�ϵ������ʶ��Զ����ӷ�����ɫ��ѧ����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com