
����Ŀ��
(��)��֪��ҵ������H2�Ĺ����������·�Ӧ�� CO (g) + H2O (g)![]() CO2(g) + H2 (g)��һ�������µ��ܱ������У��÷�Ӧ�ﵽ��ѧƽ��״̬����ش�
CO2(g) + H2 (g)��һ�������µ��ܱ������У��÷�Ӧ�ﵽ��ѧƽ��״̬����ش�
(1)������H2O (g)��Ũ�ȣ���CO��ת���� _________�������С������
(2)�������¶���ʹ��ѧƽ��������Ӧ�����ƶ���������Ӧ��____________������ȡ������ȡ�����Ӧ��
(��)úȼ�յķ�Ӧ�ȿ�ͨ����������;�������ã�a.����ú�ڳ���Ŀ�����ֱ��ȼ�ղ����ķ�Ӧ�ȣ�b.��ʹú��ˮ������Ӧ�õ�������һ����̼��Ȼ��ʹ�õ���������һ����̼�ڳ���Ŀ�����ȼ�ա����������̵Ļ�ѧ����ʽΪ��
a.C(s)��O2(g) �T CO2(g)����H�T E1 ��
b.C(s)��H2O(g) �T CO(g)��H2(g)����H�T E2 ��
H2(g)��1/2 O2(g) �TH2O(g)����H�T E3 ��
CO(g)��1/2 O2(g) �TCO2(g)����H�T E4 ��
��ش�
����;��a��ȣ�;��b�н϶���ŵ㣬��____________��
�������ĸ��Ȼ�ѧ����ʽ���ĸ���Ӧ�ġ�H��0 ��_____________��
�ǵ�������ú�ֱ�ͨ������������ͬ��;�������Ŀ����õ���������ϵ��ȷ������______��
A.a��b�� B.a��b�� C.a��b����������ͬ
�ȸ��������غ㶨�ɣ�E1 ��E2�� E3��E4֮��Ĺ�ϵΪ________________________��
���𰸡� ���� ���� ��̬ú�������������ȼ�Ϻ������Դ�����SO2���̳��Կ�����ɵ���Ⱦ������ȼ��Ч�ʸߣ�Ҳ�������� �� C E1 �TE2 ��E3 ��E4
����������I����1������Ӧ��ˮ������Ũ�ȣ�ƽ��������Ӧ�����ƶ�����CO��ת�������ʴ�Ϊ������
��2�������¶�ƽ�������ȷ�Ӧ�����ƶ����������¶���ʹ��ѧƽ��������Ӧ�����ƶ���˵������Ӧ�����ȷ�Ӧ���ʴ�Ϊ�����ȣ�
(��)��1������ú�������������ȼ�Ϻ������Դ�����SO2���̳��Կ�����ɵ���Ⱦ������ȼ��Ч�ʸߣ�Ҳ�������䣬�ʴ�Ϊ����̬ú�������������ȼ�Ϻ������Դ�����SO2���̳��Կ�����ɵ���Ⱦ������ȼ��Ч�ʸߣ�Ҳ�������䣻
��2���٢ۢ�Ϊȼ�շ�Ӧ�����Է��ȷ�Ӧ����Ϊ���ȷ�Ӧ���ʴ�Ϊ������
��3���ɸ�˹���ɿ�֪����Ӧһ����ɻ�ֲ���ɣ�����ЧӦ��ͬ�����������ú�ֱ�ͨ������������ͬ��;�������Ŀ����õ���������ͬ���ʴ�Ϊ��C��
��4���ɸ�˹���ɿ�֪����=��+��+�ܣ�������ΪE1=E2+E3+E4���ʴ�Ϊ��E1=E2+E3+E4��


 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
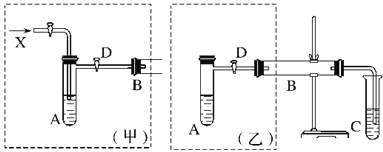
����Ŀ�� ���Ȼ���(ICl3)��ҩ��ϳ�����;�dz��㷺�����۵㣺33�����е㣺73����ʵ���ҿ�����ͼ��ʾװ����ȡICl3 ��

��1������a �������� ��
��2���Ʊ�����ѡ�õ�ҩƷΪƯ�۹���[��Ҫ�ɷ�ΪCa(ClO)2]��Ũ���ᣬ�йط�Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��B(����ƿ)�������ڳ��ӣ�Ҳ�ǰ�ȫƿ���ܼ��ʵ�����ʱװ��C���Ƿ����˶�������������������ʱB������ ��
��4���Լ�XΪ ��
��5�������뵥�ʵ������¶��Ե���70���·�Ӧ����װ��D���˵ļ��ȷ�ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҽ�����̷���FeSO47H2O��������ȱ����ƶѪ����Чҩ��ij��ѧ��ȤС����̷����������µ�̽����

���Ʊ���Ʒ����С���ɷ���м�����������ۡ�����ͭ�������������ʣ�������ͼ��ʾװ���Ʊ�FeSO47H2O���壬�������£�
��1��Ԥ�������Ƚ�����м���뵽����Na2CO3��Һ��ϴ�ӣ�Ŀ����_________________��Ȼ����м��ˮϴ��2��3�顣
��2����ϴ�Ӻ�ķ���м���뵽Բ����ƿ�С���ϡ������з�ӦǰҪ����ͨ��N2��ͨ��N2��������____________________��
��3����������ϡ���ᣬ�����¶�50�桫80��֮�䣬��ַ�Ӧ��Բ����ƿ��ʣ��Ĺ���Ϊ_________��
��4����ȡ��Ʒ�������裨3���з�Ӧ��Ļ������������������ˮ�����ȹ��ˡ�����Һ____________���˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ��ܱձ����Ʒ��
���ⶨFeSO47H2O������
��1����ȡ������Ʒ10.0g������������ϡ�����У����100mL��Һ����Ҫ����������ƽ�����������ձ�����Ͳ�⣬����Ҫ�������У����������ƣ�___________��_____________��
��2������Һ��ȷ��ȡ25.00mL��Һ������ƿ�У���0.1000mol/LKMnO4����Һ�ζ�����ζ��յ���жϷ�����__________________________________��
��3����ͬ���ķ����ζ�3�Σ�ƽ������10.00mL��Һ������Ʒ��FeSO47H2O����������Ϊ_________������֪Mr��FeSO47H2O��=278����
��4�����������ƫС�����������ñ���Һ�ζ�ʱ������___������ţ��������¡�
A����ƿ����ˮϴ��δ���Ҳδ�ô���Һ��ϴ
B����ʽ�ζ���δ�ñ�Һ��ϴ��ֱ������ʢװ����Һ
C���ζ��յ�ʱ�����Ӷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A���ʾ�����ͼ�ı仯���̣�

��֪��
��1��A��ϵͳ��������������__________________
��2��A�ڴ��������¿�������H2��Ӧ����B���÷�Ӧ�ķ�Ӧ������____________�����
����C�Ľṹ��ʽ��________________________________________��
��3�������ᣨ  ���ж���ͬ���칹�壮д������һ�֣�Ҫ��������������ȡ����������FeCl3��Һ������ɫ��Ӧ��Ҳ����NaHCO3��Һ��Ӧ�ų������ͬ���칹��Ľṹ��ʽ____________________��
���ж���ͬ���칹�壮д������һ�֣�Ҫ��������������ȡ����������FeCl3��Һ������ɫ��Ӧ��Ҳ����NaHCO3��Һ��Ӧ�ų������ͬ���칹��Ľṹ��ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ǰ�����ڵ�A��B��C��D��E��F��G����Ԫ�أ�ԭ��������������AԪ�صļ۵��ӹ���Ϊnsnnpn+1��CԪ��Ϊ����õķǽ���Ԫ�أ�DԪ�غ������������Ӳ㣬�����������Ǻ������������1/6��EԪ�����������ӵ�3d���Ϊ�����״̬��FԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�GԪ����AԪ��λ��ͬһ���壬��ij���������о綾��
��1��AԪ�صĵ�һ������________BԪ�صĵ�һ�����ܣ��<����>����=������
��2��DԪ�ػ�̬ԭ�Ӻ������ռ�ݵ�����ܲ����Ϊ________�� DC2�ĵ���ʽΪ__________��
��3��Eԭ�Ӽ۵��ӵĹ����ʾʽΪ_____________________________��
��4��F���̬ԭ�ӵĵ����Ų�ʽΪ_____________________��Ԫ��λ��Ԫ�����ڱ���_______����
��5��GԪ�ؿ��ܵ�������___________������ţ���
A���䵥�ʿ���Ϊ�뵼�����
B����縺�Դ�����
C������������Ӧ��ˮ������ǿ��
��6����A��B��Ԫ�صĻ�����Ľṹ��ͼ1��ʾ���û������к���_________������ţ���
a�����Թ��ۼ� b���Ǽ��Թ��ۼ� c����λ�� d����� e��������
��7��A��E�γɵ�һ�ֻ�����ľ����ṹ��ͼ2��ʾ���þ����Ļ�ѧʽΪ_____________�������������Eԭ�Ӽ�ľ���Ϊacm����þ�����ܶ���________________g��cm-3�����������ͣ��ʾ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҿ������Ҷ�����ͭ��ͭ�Ļ������Ʊ��Ҷ�ȩ����ͼ��ij��ȤС����Ƶ�ʵ��װ�����ұߵķ�Ӧװ����ͬ������ߵ����巢��װ�ò�ͬ���Թ�C��װ��ˮ(����װ��δ����)����ش�����������
(1)����װ���е�A��B�����������A����ˮԡ������A����ˮԡ���ȵ���Ҫ�ŵ���____________________________��
(2)������װ�ý���ʵ����B�ܴ�װͭ����ͨ��A�ܵ�����ΪX��B�з�Ӧ�Ļ�ѧ����ʽΪ
_________________________________________________________��
(3)������װ�ý���ʵ������B����Ӧװ___________________________________________��
B�з�Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________��
(4)ʵ�����ʱ�ȴ�D���Ļ������ٳ�ȥ�ƾ�������������Ŀ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��������ǻ��������������������Ӧ�ù㷺��

��1��P4S3����������������ӽṹ��ͼ1��ʾ��
��P4S3��������ԭ�ӵ��ӻ��������Ϊ ________________��
��ÿ��P4S3�����к��µ��ӶԵ���ĿΪ_________________��
��2�������۵�Ϊ2000�棬���뾧��軥Ϊ�ȵ����壬���������ṹ��ͼ2��ʾ��
�����������������������������Ϊ_______________��
��ͼ��A���B���ԭ�����������ͼ��ʾ�� ��C���ԭ���������Ϊ_____________��
��3��Fe3+��Co3-��N3-��CN-�ȿ��γ�������ӡ�
��C��N��O�ĵ�һ����������Ϊ____________����ԭ����________________________________��
��K3[Fe(CN)6]�����ڼ���Fe2+�� lmol[Fe(CN)6]3-�����к��ЦҼ�����ĿΪ__________________��
��[Co(N3)(NH3)5]SO4��Co����λ��Ϊ_____________��
��4��������FeF3�۵����1000�棬��Fe(CO)5���۵�ȴ����0��, FeF3�۵�Զ����Fe(CO)5�Ŀ���ԭ����__________________________________________________��
��5��ij�ִ��Ե���������Ľṹ��ͼ 3��ʾ���û�����Ļ�ѧʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. ������ˮʱ��������������ѧ�仯����������ɱ������������
B. ϡ������������Һ����������Һ���ܲ��������ЧӦ
C. �챦ʯ������ʯ��Ҫ�ɷ�������������ʯӢ����������ɸ����Ҫ�ɷ��ǹ�����
D. �ճ������м��(Na2CO3��10H2O)��ɼ���(Na2CO3)���ڷ绯���������仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ����٤��������ֵ������˵����ȷ���ǣ�������
A. lmol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA
B. ����������ˮ��Ӧʱ������0.1mol����ת�Ƶĵ�����Ϊ0.2NA
C. 18gD2O��18gH2O�к��е���������Ϊ10NA
D. 1 mol Na2O2�����к���������Ϊ4NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com