
�����������ӻ�����A��B��C��D��E���������±��������γɵģ�
| ������ | Ag�� | Ba2�� | Al3�� |
| ������ | OH�� | Cl�� | SO |
Ϊ�������ǣ��ֱ��������ʵ�飬�����ǣ�
a��B��D��������ˮ��Ҳ�������
b��A����ˮ��������ij�����ӷ�Ӧ������B����A��Һ�������ˮ��Ӧ���ɰ�ɫ������
c��C����ˮ��������ij�����ӷ�Ӧ������D����C��Һ�������ˮ��Ӧ���ɰ�ɫ������
d��E����ˮ��������ij�����ӷ�Ӧ������B��
e��A��Һ������E��Һ��Ӧ���ɳ������ټӹ���E��Һ�����������٣�������ʧ��
���������ʵ��������գ�
(1)д��������Ļ�ѧʽ��A________��C________��D________��E________��
(2)A��Һ�����E��Һ��Ӧ�����յõ��ij����Ļ�ѧʽ��______________��
��������������ؼ����ҵ�ͻ�ƿڣ�Ȼ��˳�����ϣ������Ƴ�������ʡ�������������ɵij����ij������У�AgCl��Al(OH)3��BaSO4������AgCl��BaSO4�������ᡣʵ��e�DZ����ͻ�ƿڣ����ټӹ���E��Һ�����������١���˵��E��Һ���ܽ�ijһ�ֳ�������������Eֻ����һ��ǿ����ܽ�ij���Ҳֻ����Al(OH)3�����E����������OH������������ֻ����Ba2��(��ΪOH����Ag����Al3���ܷ�Ӧ)����EΪBa(OH)2�����A����������Al3�������A��������ֻ����SO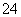 ʱ���ŷ��ϡ����������٣�������ʧ������AΪAl2(SO4)3������������ʵ��b��d֪BΪBaSO4����DΪ��һ�ֲ�������ij���AgCl������ʵ��c�����µ����ӿ��ж�CΪAlCl3��
ʱ���ŷ��ϡ����������٣�������ʧ������AΪAl2(SO4)3������������ʵ��b��d֪BΪBaSO4����DΪ��һ�ֲ�������ij���AgCl������ʵ��c�����µ����ӿ��ж�CΪAlCl3��
���𰸡���(1)Al2(SO4)3��AlCl3��AgCl��Ba(OH)2��(2)BaSO4


 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ����
A��Һ���ӷ����ڴ��Һ����Լ�ƿ��Ӧ��ˮ��
B����ʹ��ʪ�ĵ���KI��ֽ�����ɫ������һ����Cl2
C��ij��Һ����CCl4��CC14������ɫ��֤��ԭ��Һ�д���I−
D��ij�ܱ�����BaCl2��Һ������������ϡ����İ�ɫ����������Һһ������Ag��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʼ��仯�����й㷺Ӧ�á�
��1������ʯ����Ҫ�ɷ�Ca5��PO4��3F���ڸ������Ʊ����ף�P4�����Ȼ�ѧ����ʽΪ��4Ca5��PO4��3F��s��+21SiO2��s��+30C��s��3P4��g��+20CaSiO3��s��+30CO��g��+SiF4��g�� ��H
��������Ӧ�У����������������___________________________________��
����֪��ͬ�����£�
4Ca5��PO4��3F��s��+3SiO2��s��====6Ca3��PO4��2��s��+2CaSiO3��s��+SiF4��g�� ��H1
2Ca3��PO4��2��s��+10C��s��====P4��g��+6CaO��s��+10CO��g�� ��H2
SiO2��s��+CaO��s��====CaSiO3��s�� ��H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��_____________________________��
��2�������������Ϊ����������ӣ�����ṹʽ����ͼ��֮����ȥ����ˮ���ӵIJ����ṹʽΪ________________________�����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ___________________________��

��3���������ƣ�NaH2PO2�������ڻ�ѧ������
��NaH2PO2��PԪ�صĻ��ϼ�Ϊ________________________��
�ڻ�ѧ��������Һ�к���Ni2+��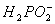 �������Ե������·���������Ӧ��
�������Ե������·���������Ӧ��
(a)__________Ni2++__________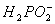 +__________
+__________ __________Ni+________
__________Ni+________ +_______
+_______
��b��6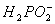 +2H+====2P+4
+2H+====2P+4 +3H2��
+3H2��
���������д������ƽ��Ӧʽ��a����
�����â��з�Ӧ�������϶Ƽ�����������Ͻ𣬴Ӷ��ﵽ��ѧ������Ŀ�ģ�����һ�ֳ����Ļ�ѧ�ơ�������·���Ƚϻ�ѧ�����ơ�
�����ϵIJ�ͬ�㣺_______________��ԭ���ϵ���ͬ�㣺_______________����ѧ�Ƶ��ŵ㣺_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�Cl��N��S�ȷǽ���Ԫ�ػ������˵����ȷ���ǡ��� ��
A.Ư�۵ijɷ�Ϊ�������
B.ʵ���ҿ���Ũ������ﰱ��
C.ʵ���ҿ���NaOH��Һ����NO2��HCl����
D.Al2(SO4)3�ɳ�ȥ���Է�ˮ�����Է�ˮ�е���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵζ���������������ȷ����(����)
A�����ζ�����Һ����20.00 mL������Һ��ȫ������ʱ����Һ�����Ϊ20.00 mL
B���ñ�NaOH��Һ�ζ�CH3COOH��Һʱ����ѡ�ü���Ϊָʾ��
C���ñ�������Һ�ζ�δ֪Ũ��NaOH��Һʱ�����ζ�ǰ�ζ��ܼ��촦������δ�ų����ζ�����ʧ������ʹ������ƫ��
D���ζ�ʱ�ɽ�KMnO4��Һװ�ڼ�ʽ�ζ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о����֣�������������NO2�ܲ���������������γɣ���Ӧ�������£�
 ��SO2+NO2
��SO2+NO2 SO3+NO
SO3+NO
��SO3+H2O H2SO4
H2SO4
��2NO+O2 2NO2
2NO2
NO2�����������е����ã���H2SO4�������仯�е��������Ƶ��ǣ� ��
A.��ʪ������ͨ��ʢ��ŨH2SO4��ϴ��ƿ B.����ͨ��ŨH2SO4��
C.ŨH2SO4����өʯ�У����� D.��������H2SO4ʹ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪:X+Y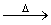 Z+W
Z+W
(1)Y�ĵ���ʽ��_________________________��
(2)Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ��_________________________________��
(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
a.����ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b.ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
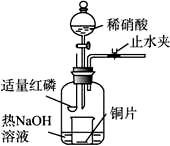
�ٲ���c��ȱ�ٵ�һ����Ҫ������_______________________________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ______________________________________��
�۲���c����ϡ������ձ��е�������______________________________________
____________________________________________________________________��
��Ӧ�����ӷ���ʽ��____________________________________________________��
(4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2������ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
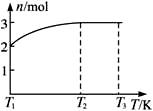
���¶���T1��T2֮�䣬��Ӧ�Ļ�ѧ����ʽ��_________________________��
���¶���T2��T3֮�䣬�����ƽ����Է��������ǣ�����1λС����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
20���Ϳ�ѧ���ٷ�չ������������������δ������д���ѧ��ѧ�����������( )
A.������ӽṹ��ȷ�� B.��¯����
C.���³�ѹ�ϳɰ� D.�ɳ��ɵ�ص�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y��Z���Ƕ����ڵ�Ԫ�أ����ǵ�ԭ���������ε�����Xԭ�ӵĵ��Ӳ��������ĺ������������ͬ����Zԭ�ӵ������������Ǵ�����������Y��Z�����γ�����������̬�������
��1��X�� ��Y�� ��Z�� ��
��2����Y��Z��ɣ���Y��Z��������Ϊ7��20�Ļ�����Ļ�ѧʽ������ʽ���� ��
��3����X��Y��Z�е�����Ԫ����ɣ�����X2Z���Ӿ�����ͬ������������������ �� ��
��4��X��Y��Z�����γ�һ���Σ�������X��Y��ZԪ�ص�ԭ�ӵĸ�����Ϊ4��2��3�����εĻ�ѧʽ������ʽ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com