


 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
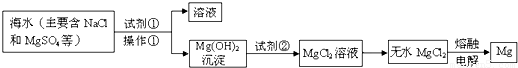
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
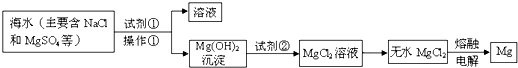
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡģ���� ���ͣ�ʵ����
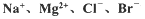 �ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
�ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺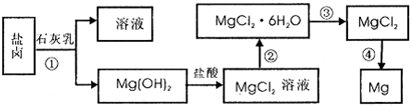

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
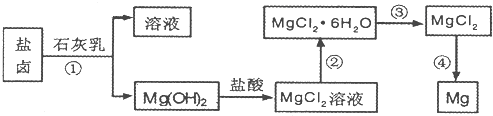
þ����Ͻ�����;�㷺�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ �ġ�ijѧУ������ȤС��Ӻ�ˮɹ�κ����±����Ҫ��Na+��Mg2+��Cl-��Br-�ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
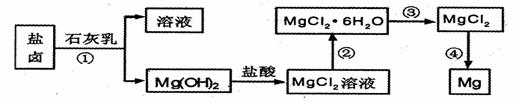
��1����ת�����ٵõ���Mg�� OH��2�����л���������Ca�� OH��2����ȥ����Ca�� OH��2�ķ������Ƚ��������뵽ʢ�� ���ձ��У���ֽ���� __ __��������������ɵô�����Mg�� OH��2���ڴ˲��������У��������������ǽ���� .
��2��д��ת�����з�����Ӧ�Ļ�ѧ����ʽ___ _��
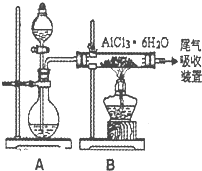
��3����֪ת���۵ķ�Ӧԭ������ȡ��ˮAlCl3��ͬ��ͼ����ȡ
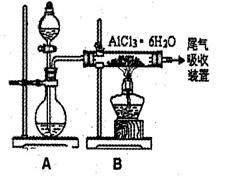 ��ˮAlCl3ʵ��װ��ͼ��װ��A�е���Һ��ֱ���
��ˮAlCl3ʵ��װ��ͼ��װ��A�е���Һ��ֱ���
Ũ�����Ũ���ᡣ��ش�
��DΪʲô��ֱ�Ӽ�������ȡ��ˮAlCl3�����û�ѧ
����ʽ��ʾ�� ��
�ڷ�Һ©����Ӧʢװ���Լ���__ __��
���ɷ�Һ©������ƿ�м��Լ�ʱӦע�������
�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com