
��Ӧ2C��O2===2CO�������仯����ͼ��ʾ������˵����ȷ����(����)
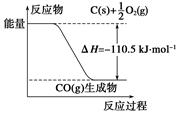
A��12 g C(s)��һ����O2(g)��Ӧ����14 g CO(g)�ų�������Ϊ110.5 kJ
B���÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)��O2(g)===2CO(g)����H����221 kJ
C��2 mol C(s)������O2(g)��Ӧ����CO2(g)���ų�����������221 kJ
D���÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������O2�����л�ѧ������ʱ�����յ��������IJ�
C
��������
�������������ͼʾ��Aѡ�����12 g C(s)��һ����O2(g)��Ӧ����28 g CO(g)�ų�������Ϊ110.5 kJ��Bѡ�����H�ĵ�λһ��ΪkJ��mol��1��kJ/mol�����÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)��O2(g)===2CO(g) ��H����221 kJ��mol��1��Cѡ����ȷ��2 mol C(s)��O2(g)��Ӧ����CO(g)�ų�������Ϊ221 kJ������CO(g)��O2(g)��Ӧ����CO2 (g)���ȣ����2 mol C(s)������O2 (g)��Ӧ����CO2(g)�ų�����������221 kJ��Dѡ����÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������C��O2�����л�ѧ������ʱ�����յ��������IJ��ѡC��
���㣺��ѧ��Ӧ�������仯
������ע���Ȼ�ѧ����ʽ����д�����ж��Լ���˹���ɵ�����,���ڳ���֪ʶ�㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013�꺣�������λ���ѧ�߶��ϸ��н�ѧ�����������ѧ�Ծ����������� ���ͣ���ѡ��
��Ӧ2C��O2===2CO�������仯����ͼ��ʾ������˵����ȷ����(����)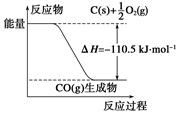
| A��12 g C(s)��һ����O2(g)��Ӧ����14 g CO(g)�ų�������Ϊ110.5 kJ |
| B���÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)��O2(g)===2CO(g)����H����221 kJ |
| C��2 mol C(s)������O2(g)��Ӧ����CO2(g)���ų�����������221 kJ |
| D���÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������O2�����л�ѧ������ʱ�����յ��������IJ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com