
(15��)��ͼ������A��M��һ�������µ�ת����ϵ(���ֲ��P��Ӧ����δ�г�)�����У�I�ǵ�������ԭ�Ӱ뾶��С�Ľ���Ԫ����ɵĵ��ʣ�D��һ�ֺ���ɫ�����ĩ��GΪ���ʣ�K��һ�ֺ���ɫ���壬A��B��Է����������16��J��C���dz�����ǿ�ᡣ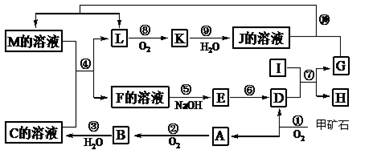
����д���пհף�
��1��д���������ʵĻ�ѧʽB E ��
��2����Ӧ�ߵĻ�ѧ����ʽΪ ����Ӧ������ ��
A.���ȷ�Ӧ B.���ȷ�Ӧ C.�û���Ӧ D.������ԭ��Ӧ
��3����Ӧ������ӷ���ʽΪ ��
��4����������D��KNO3��KOH��ϣ��ڸ��������¿��Ƶ�һ�֡���ɫ��������Ч��ˮ��K2GO4(G��+6��)��ͬʱ������KNO2��H2O���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��5����Ӧ�������ɵ�A��D���ʵ���֮��Ϊ4:1����֪��ʯ����Ҫ�ɷ���������Ԫ����ɵĻ������û�����Ļ�ѧʽΪ ��
��1��B SO3 E Fe(OH)3�� ��2�� Fe2O3+ 2Al Al2O3+2Fe ����Ӧ������ BCD ��
Al2O3+2Fe ����Ӧ������ BCD ��
��3�� 3Fe��2NO3�� ��8H�� = 3Fe2����2NO����4H2O ��
��4�� Fe2O3��3KNO3��4KOH  2K2FeO4��3KNO2��2H2O����5�� FeS2��
2K2FeO4��3KNO2��2H2O����5�� FeS2��
���������������1����ʯ��FeS2,��ʯ���յõ�A��SO2��D��Fe2O3��SO2��O2������Ӧ�õ�B��SO3��SO3��ˮ���õõ�C�����I��Al,Al��Fe2O3�������ȷ�Ӧ��Fe2O3+ 2Al Al2O3+2Fe���õ���G��Fe;H��Al2O3��E��Fe(OH)3��F��Fe2(SO4)3��Fe(NO3)3�Ļ���M��Fe(NO3)2.L��NO��K��NO2��J�� HNO3����1��B��SO3��E��Fe(OH)3����2����Ӧ�ߵ����ȷ�Ӧ�Ļ�ѧ����ʽΪFe2O3+ 2Al
Al2O3+2Fe���õ���G��Fe;H��Al2O3��E��Fe(OH)3��F��Fe2(SO4)3��Fe(NO3)3�Ļ���M��Fe(NO3)2.L��NO��K��NO2��J�� HNO3����1��B��SO3��E��Fe(OH)3����2����Ӧ�ߵ����ȷ�Ӧ�Ļ�ѧ����ʽΪFe2O3+ 2Al Al2O3+2Fe�����ȷ�Ӧ�Ƿ��ȷ�Ӧ����Ϊ�����û���Ӧ�Ķ��壬���������û���Ӧ����Ԫ�صĻ��ϼ۵������뽵�ͣ�����Ҳ��������Ӧ��Ӧ�����ѡ����B��C��D����3��������Fe�����ᷴӦ�����ӷ���ʽ��3Fe��2NO3�� ��8H�� = 3Fe2����2NO����4H2O����4����������ɵ���Ӧ�ķ�Ӧ�ķ���ʽ�ǣ�Fe2O3��3KNO3��4KOH
Al2O3+2Fe�����ȷ�Ӧ�Ƿ��ȷ�Ӧ����Ϊ�����û���Ӧ�Ķ��壬���������û���Ӧ����Ԫ�صĻ��ϼ۵������뽵�ͣ�����Ҳ��������Ӧ��Ӧ�����ѡ����B��C��D����3��������Fe�����ᷴӦ�����ӷ���ʽ��3Fe��2NO3�� ��8H�� = 3Fe2����2NO����4H2O����4����������ɵ���Ӧ�ķ�Ӧ�ķ���ʽ�ǣ�Fe2O3��3KNO3��4KOH  2K2FeO4��3KNO2��2H2O����5����Ӧ�������ɵ�SO2��Fe2O3���ʵ���֮��Ϊ4:1����֪��ʯ����Ҫ�ɷ���������Ԫ����ɵĻ������û�������n(Fe):n(S)=2:4=1:2��˼Ļ�ѧʽΪFeS2��
2K2FeO4��3KNO2��2H2O����5����Ӧ�������ɵ�SO2��Fe2O3���ʵ���֮��Ϊ4:1����֪��ʯ����Ҫ�ɷ���������Ԫ����ɵĻ������û�������n(Fe):n(S)=2:4=1:2��˼Ļ�ѧʽΪFeS2��
���㣺�������ʵ��ƶϡ����ʡ��ת�������ʵĻ�ѧʽ����ѧ����ʽ����д��֪ʶ��


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ����ȡ��ˮ�Ȼ�ͭ��ʵ��װ��ͼ����Ũ����μӵ�ʢ�ж������̷�ĩ��Բ����ƿ�С���ش��������⣺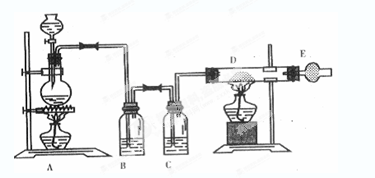
(1)ʢ��Ũ�������������Ϊ�ߣߣߣ���
(2)��ƿ�з�����Ӧ�Ļ�ѧ����ʽ ��
(3)Cƿ�е��Լ����ߣߣߣ������������ߣߣߣ���
(4)������D�з�����Ӧ�Ļ�ѧ����ʽ ����Ӧ�������ߣߣ���
(5)�����E��ʢ�м�ʯ��(CaO+NaOH)�����������ߣߣߣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧ��ȤС���ij������ʯ����Ҫ �ɷ�ΪFeS2)������Ԫ�غ����ⶨ��ʵ��̽������ҵ���������̽����
I ����m1,g�û�������Ʒ�������в������������������ͼ��ʾװ�ã��гֺͼ���װ�� ʡ�ԣ���ʯӢ����,��a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2 + 11O2 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2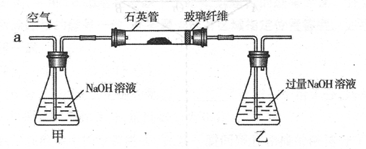
II��Ӧ������,����ƿ�е���Һ�������´���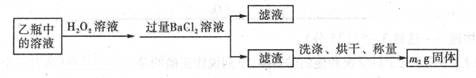
��1��I�У���ƿ�ڷ�����Ӧ�����ӷ���ʽ��____________��__________��
��2��II�У�����H2O2��Һ��������������____________________________
��3���û�����ʯ����Ԫ�ص���������Ϊ____________________________
��4�������ڴ���Ӧ���������Ƚ�������Ŀ�ģ�______________��
��5����ҵ�����г��ð��������ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ��,�� ������ѧ����ʽ��ʾ�䷴Ӧԭ��_______________��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ��Ӧ����ͼ��ʾ��װ�����о����ʵ����ʣ���������X����Ҫ�ɷ��������������ǿ�����ˮ����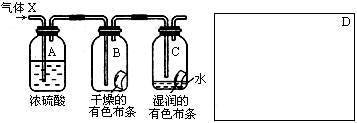
�ش��������⣺
(1)�����о�(ʵ��)����ҪĿ���� ��
(2)ŨH2SO4�������� �����о�Ŀ��ֱ����ص�ʵ�������� ��
(3)��ʵ��װ������ϴ��ڵ�ȱ��Ϊ ��������ͼ��D�������ܿ˷���ȱ�ݵ�װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij�����ĩ���п��ܺ���K2CO3��KNO3��NaNO2��K2SO3��Na2SO4��FeO��Fe2O3�е������֣�ijͬѧΪȷ���ù����ĩ�ijɷ֣�ȡ��������ʵ�飬ʵ����̼��������£� 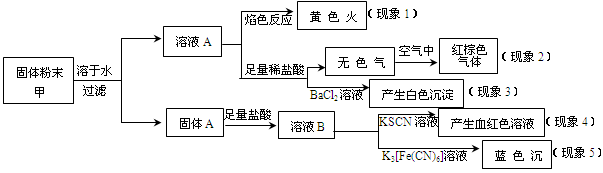
��ͬѧ�ó��Ľ�����ȷ����
| A����������1���Ƴ��ù����ĩ�к�����Ԫ�أ���������Ԫ�� |
| B����������2���Ƴ��ù����ĩ��һ������NaNO2 |
| C����������3���Ƴ��ù����ĩ��һ������Na2SO4 |
| D����������4������5���Ƴ��ù����ĩ��һ������FeO��Fe2O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪����ת����ϵ��M��N��Ϊ���ʣ���M��������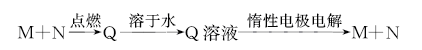
| A��Na | B��Cu | C��H2 | D��Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ����ͼ��ʾ�����ֲ��P��Ӧ��������ȥ������֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壻MΪ���ɫ������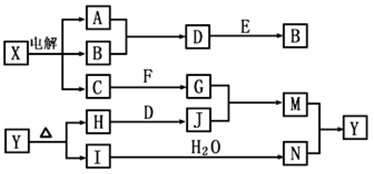
��ش��������⣺
��1��B������Ԫ��λ�����ڱ��е� ���ڣ� �塣
��2��A��B��ȼ�յ������� ��
��3��D+E��B�ķ�Ӧ�У��������뱻��ԭ�����ʵ����ʵ������� ��
��4��G+J��M�����ӷ���ʽ�� ��
��5��Y���ȷֽ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com