
��֪Zn(OH)2������NaOH��Һת���Na2[Zn(OH)4]��Ϊ�˲ⶨij����Ƥ��п��������������������ʵ�飺ȡa g����Ƥ��Ʒ���ձ��У��������ϡ���ᣬ�ñ�����Ǻã���ʼʱ�������ݵ��ٶȺܿ죬�Ժ�������������ȫ���ܽ�����ձ��м������NaOH��Һ����ֽ������ˣ������ó����ڿ����м�ǿ�����������䣬�Ƶò�����������Ϊb g��
(1)����Ƥ��п��Ŀ����
________________________________________________________________________��
(2)���ݲ����ٶ��ȿ������ԭ����
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(3)��Һ�м������NaOH��Һ��������Ӧ�����ӷ���ʽ��(�������ӷ�Ӧ��д��ѧ����ʽ)��
________________________________________________________________________��
________________________________________________________________________��
________________________________________________________________________��
________________________________________________________________________��
(4)�������Ĺ�����__________���õ��ù���Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________��
(5)����Ƥ��п������������______________(�ú�a��b��ʽ�ӱ�ʾ)��
(1)�����ڲ��������������Ŀ���ʴ����
(2)��ʼʱ��������Ũ�ȴ���п������������С��ԭ��أ���Ӧ�ٶȿ졣���ŷ�Ӧ�Ľ��У���Һ��������Ũ�ȼ�С������п�ܽ��ԭ���������С���������ڣ���Ӧ�������Լ���
(3)Zn2����2OH��=Zn(OH)2��
Zn(OH)2��2OH��=[Zn(OH)4]2��
Fe2����2OH��=Fe(OH)2��
4Fe(OH)2��O2��2H2O=4Fe(OH)3
(4)Fe2O3��2Fe(OH)3 Fe2O3��3H2O
Fe2O3��3H2O
(5)  %
%
��������
���������(1)����Ƥ��п��Ŀ����Ϊ�˱����ڲ���Fe������Fe�Ŀ���ʴ������
(2)Zn��Fe��ϡ�������γ�ԭ��أ���Ӧ���ʿ죬����H��Ũ�ȵIJ��ϼ�С������Zn�ܽ⣬ԭ���������������Ӧ����������
(3)��������Ϣ����ѧ��ѧ֪ʶ������д��(3)�е����ӷ���ʽ��
(4)Fe(OH)3���ȶ�������ʱ�����ֽ⣬���Եõ��Ĺ�����Fe2O3��
(5)���ݲ�������������������b g�ɵ���Ԫ�ص�����Ϊ��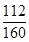 b g������a g����Ƥ��п������Ϊ(a��
b g������a g����Ƥ��п������Ϊ(a��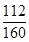 b) g���Ӷ����Լ����п������������
b) g���Ӷ����Լ����п������������ %��
%��
���㣺�������Ƥ��п�����������ⶨ��ʵ����ơ������������Լ�������ѧ�������д
�����������Բⶨij����Ƥ��п����������Ϊ���壬�ص㿼����ѧ���淶�Ͻ���ʵ����ƺͶ��ֲ����������Լ��淶�Ĵ�������������������ѧ����ѧ������������ѧ����ѧϰ��Ȥ�����ѧ����Ӧ��������ѧϰЧ�ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���㶫ʡ�����з�خ��2010��������Ĵ�ͳ�����ۻ�ѧ���� ���ͣ������
п������һ�ְ�ɫ���ϡ���ҵ������ZnSO4��BaS��Һ��϶��ɣ�BaS+ZnSO4 = ZnS��+BaSO4���������ǹ�ҵ�������̡���ش��й����⣺
��.ZnSO4��Һ���Ʊ����ᴿ
�й����ϣ���֪Zn(OH)2��Al(OH)3���ƣ������ڹ�����NaOH��Һ����Na2ZnO2��
��п�����Ҫ�ɷ���ZnCO3��������SiO2��FeCO3��Cu2(OH)2CO3�ȡ�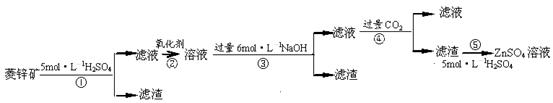 ��1������ʹ�õ���������������е� ������ţ��������� ��
��1������ʹ�õ���������������е� ������ţ��������� ��
| A��Cl2 | B��H2O2 | C��KMnO4 | D��ŨHNO3 |
 �й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ?mol-1
�й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ?mol-1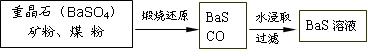
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���㽭ʡ�����и�����һ����Ӧ�Բ������ۻ�ѧ�Ծ��������棩 ���ͣ������
��������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɻ�ͭ(Cu��Zn�Ͻ𣬺���������Fe)�Ʊ���������(CuSO4��5H2O)��������ZnO���Ʊ�����ͼ���£�
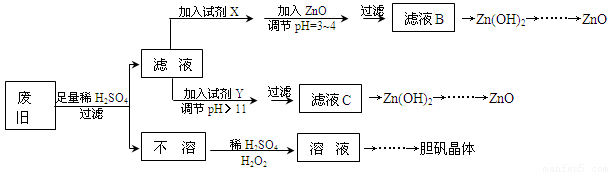
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L��1����)��
| Fe3�� | Fe2�� | Zn2�� |
��ʼ������pH | 1.1 | 5.8 | 5.9 |
������ȫ��pH | 3.0 | 8.8 | 8.9 |
��ش��������⣺
��1���Լ�X������__________����������____________________��
��2������ZnO����pH=3��4��Ŀ����____________________��
��3���ɲ�����������ҺD�Ļ�ѧ����ʽΪ______________________________��
��4������ҺD�Ƶ��������������Ҫ����������______________________________��
��5�������Լ�����ΪY�Լ�����______��
A��ZnOB��NaOHC��Na2CO3D��ZnSO4
������ҺC����μ�������ֱ���������������������______________________________��
��6���ⶨ��������Ĵ���(��������I��������Ӧ������������)��ȷ��ȡ0.5000g��������������ƿ�У�������ˮ�ܽ⣬�ټ������KI����0.1000mol��L��1Na2S2O3����Һ�ζ����յ㣬����Na2S2O3����Һ19.40mL����֪�������ζ������е����ӷ���ʽ���£�
2Cu2����4I�� 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32�� 2I����S4O62��
2I����S4O62��
����������Ĵ���Ϊ_______________��
���ڵζ������о���ҡ��(��Һ���⽦)��ƿ��������õĴ��Ƚ���__________(����ƫ��������ƫ��������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п������һ�ְ�ɫ���ϡ���ҵ������ZnSO4��BaS��Һ��϶��ɣ�BaS+ZnSO4 = ZnS��+BaSO4���������ǹ�ҵ�������̡���ش��й����⣺
��.ZnSO4��Һ���Ʊ����ᴿ
�й����ϣ���֪Zn(OH)2��Al(OH)3���ƣ������ڹ�����NaOH��Һ����Na2ZnO2��
��п�����Ҫ�ɷ���ZnCO3��������SiO2��FeCO3��Cu2(OH)2CO3�ȡ�
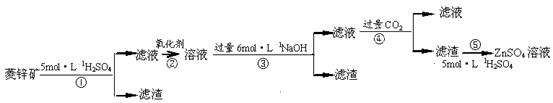 ��1������ʹ�õ���������������е� ������ţ��������� ��
��1������ʹ�õ���������������е� ������ţ��������� ��
A.Cl2 B.H2O2 C.KMnO4 D.ŨHNO3
��2��д����Ӧ�ܵ����ӷ���ʽ�� ��
��3��Ϊ�˴ﵽ�ۺ����á����ܼ��ŵ�Ŀ�ģ����������в��� ������ �������ڲ�
�� �����в���ѡ��١��ڡ��ۡ��ܡ��ݣ���
��.BaS��Һ���Ʊ�
![]() �й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ??mol-1
�й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ??mol-1
C��s���� ��O2��g����CO��g���� ��H = ��110.5 kJ??mol-1
Ba��s���� S��s����BaS��s���� ��H = ��460 kJ??mol-1
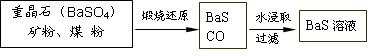
��4�����ջ�ԭ���Ȼ�ѧ����ʽΪ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���½��������¿��� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com