
����Ŀ��ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ˮ����ͭ������ɱ������
(1)Cuλ��Ԫ�����ڱ�_____�塣Cu2+����Χ�����Ų�ʽΪ_____________��
(2)CuԪ�ؿ��γ�![]() �����д��ڵĻ�ѧ��������_____________������ţ���
�����д��ڵĻ�ѧ��������_____________������ţ���
����λ�� ����� �ۼ��Թ��ۼ� �ܷǼ��Թ��ۼ� �����Ӽ�
(3)��![]() ���жԳƵĿռ乹�ͣ��ҵ����е�����NH3������Clȡ��ʱ���ܵ����ֲ�ͬ�ṹ�IJ����
���жԳƵĿռ乹�ͣ��ҵ����е�����NH3������Clȡ��ʱ���ܵ����ֲ�ͬ�ṹ�IJ����![]() �Ŀռ乹��Ϊ____________������ĸ����
�Ŀռ乹��Ϊ____________������ĸ����
a.ƽ�������� b.�������� c.������ d.V��
(4)������ͭ��Һ����εμӰ�ˮ��������ʵ������Ϊ�ȳ�����ɫ�������������ܽ��γ�����ɫ����Һ.д������ɫ�����ܽ�����ӷ���ʽ��______________��
(5)Cu2O���۵��Cu2S��__________���������������������������ԭ��_____________��
���𰸡�IB 3d9 �٢ۢ� a ![]() �� Cu2O��Cu2S��ȣ���������ͬ�������������ĵ����Ҳ��ͬ����O2�뾶��S2�뾶С������Cu2O�ľ����ܸ����۵���ߡ�
�� Cu2O��Cu2S��ȣ���������ͬ�������������ĵ����Ҳ��ͬ����O2�뾶��S2�뾶С������Cu2O�ľ����ܸ����۵���ߡ�
��������
(1)Cuλ��Ԫ�����ڱ��ڢ�B��ԭ������Ϊ29������Cu�ĵ����Ų�ʽ��дCu2+���ӵĵ����Ų�ʽ��
(2)![]() Ϊ���ӻ�����������д�����λ���ͼ��Թ��ۼ����������д��ڼ��Թ��ۼ�����������������֮��ͨ�����Ӽ���ϣ�
Ϊ���ӻ�����������д�����λ���ͼ��Թ��ۼ����������д��ڼ��Թ��ۼ�����������������֮��ͨ�����Ӽ���ϣ�
(3)��![]() Ϊ��������ṹ�������κ����ڵ�����NH3��ȡ��������һ�֣���Ϊƽ�������νṹ���������ֲ�ͬλ�÷�����
Ϊ��������ṹ�������κ����ڵ�����NH3��ȡ��������һ�֣���Ϊƽ�������νṹ���������ֲ�ͬλ�÷�����
(4)����ͭ��Һ����εμӰ�ˮ�����ȷ������ֽⷴӦ����Cu(OH)2����������ˮ����ʱ��Cu(OH)2������İ�ˮ��Ӧ������[Cu(NH3)4]2+��ʹ��Һ����ɫ���ݴ���д��Ӧ����ʽ��
(5)Cu2O��Cu2S��Ϊ���Ӿ��壬���Ӱ뾶ԽС�����Ӽ�Խǿ����Ӧ���۵�Խ�ߡ�
(1)Cu��29��Ԫ�أ�λ��Ԫ�����ڱ��ڢ�B�壬�����Ų�ʽΪ��[Ar]3d104s1����Cuʧȥ����Cu2+�Ĺ����У����뷴Ӧ�ĵ�����������4s��3d�ϸ�һ�����ӣ���Cu2+���ӵĵ����Ų�ʽ��Ϊ��[Ar]3d9��1s22s22p63s23p63d9��Cu2+����Χ�����Ų�ʽΪ3d9��
(2)![]() Ϊ���ӻ�����������д�����λ���ͼ��Թ��ۼ����������д��ڼ��Թ��ۼ�����������������֮��ͨ�����Ӽ���ϣ���˸û������к������Ӽ�����λ�������Թ��ۼ�����ѧ������Ǣ٢ۢݣ�
Ϊ���ӻ�����������д�����λ���ͼ��Թ��ۼ����������д��ڼ��Թ��ۼ�����������������֮��ͨ�����Ӽ���ϣ���˸û������к������Ӽ�����λ�������Թ��ۼ�����ѧ������Ǣ٢ۢݣ�
(3)��![]() Ϊ��������ṹ��������������ṹ���뼸������Cu����������ԭ�ӻ�ԭ���ŵ�λ�ö����ڣ���������NH3��2��Cl-ȡ��������һ�֣���
Ϊ��������ṹ��������������ṹ���뼸������Cu����������ԭ�ӻ�ԭ���ŵ�λ�ö����ڣ���������NH3��2��Cl-ȡ��������һ�֣���![]() Ϊƽ�������νṹ��������Cl-ȡ�����λ�ÿ��������ڵ����������ϣ�Ҳ�����ڶԽ���λ���ϣ���˾ͻ������ֲ�ͬλ�ã�����
Ϊƽ�������νṹ��������Cl-ȡ�����λ�ÿ��������ڵ����������ϣ�Ҳ�����ڶԽ���λ���ϣ���˾ͻ������ֲ�ͬλ�ã�����![]() ���жԳƵĿռ乹�ͣ��ҵ����е�����NH3������Clȡ��ʱ���ܵ����ֲ�ͬ�ṹ�IJ����
���жԳƵĿռ乹�ͣ��ҵ����е�����NH3������Clȡ��ʱ���ܵ����ֲ�ͬ�ṹ�IJ����![]() �Ŀռ乹��Ϊƽ�������νṹ���ʺ���ѡ����a��
�Ŀռ乹��Ϊƽ�������νṹ���ʺ���ѡ����a��
(4)������ͭ��Һ����εμӰ�ˮ�����ȷ������ֽⷴӦ����Cu(OH)2����������ˮ����ʱ��Cu(OH)2������İ�ˮ��Ӧ������[Cu(NH3)4]2+��ʹ��Һ������ɫ����Ӧ�����ӷ���ʽΪCu(OH)2+4NH3��H2O=[Cu(NH3)4]2++2OH-+4H2O��
(5)Cu2O��Cu2S��Ϊ���Ӿ��壬���Ӱ뾶ԽС�����Ӽ�Խǿ����Ӧ���۵�Խ�ߣ����������Ӱ뾶�������Ӱ뾶С������Cu2O�ľ����ܱ�Cu2S��������Cu2O�۵�ߡ�


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС���о�����ˮ�����ķ�Ӧ����λͬѧ�ֱ����������ʵ�顣
ʵ��� | ʵ��� |
|
|
��ش�
��1��ʵ�����ʪ����������______________��
��2��ʵ����з�Ӧ�Ļ�ѧ����ʽ��__________��
��3����ͬѧ�۲쵽ʵ����г������������ݣ�ʵ�������ҺB���ֺ�ɫ��˵����ҺA�к���___________��
��4����ͬѧ�۲쵽ʵ����г������������ݣ���ʵ�������ҺBδ���ֺ�ɫ����ҺBδ���ֺ�ɫ��ԭ����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaClO��Һ����NOβ���������NO��ȥ���ʡ�����������ͬʱ��NO��ת��ΪNO3-����ת������NaClO��Һ��ʼpH����ϡ������ڣ��ı仯��ͼ��ʾ������˵����ȷ���ǣ� ��
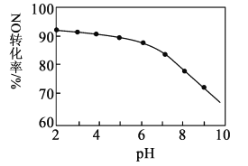
A.��Ҫ����44.8 L NO��������3 mol NaClO
B.��NaClO��Һ��ͨ��NO����ҺpH����
C.HClO����NO��������NaClOǿ
D.NaClO��Һ��c(OH-)>c(H+)+c(HClO)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ȣ�Sr3N2���ڹ�ҵ�Ϲ㷺��������ӫ��ۡ����뵪���ڼ��������¿����ɵ����ȣ���������ˮ���ҷ�Ӧ��ijͬѧ�������װ���Ʊ������ȣ���װ��ʢװ�����Լ�������ʹ�õĵ�����Ʒ���ܺ�������CO��CO2��O2���������ʡ�
��֪�������������ͭCH3COO[Cu(NH3)2]��Һ�ܶ�������CO�����ױ�O2������ʧȥ����CO�������������Ӽ�����Һ�ܶ�������O2��
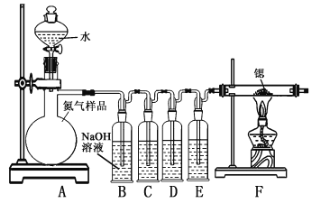
��.�����ȵ���ȡ
(1)װ��B������������_____________��
(2)װ��C��D��Eʢװ���Լ��ֱ���_____________������ţ���
�ף��������Ӽ�����Һ �ң�Ũ���� ���������������ͭ��Һ
(3)����װ����ƴ���ȱ�ݣ����ܻᵼ�²�Ʒ���ʣ�����Ľ�����____________��
��.��Ʒ���ȵIJⶨ
��ȡ6.0 g �������ò�Ʒ��������������ƿ�У�Ȼ���ɺ�ѹ©����������ˮ��ͨ��ˮ�������������İ�ȫ����������200mL1.00mol/L���������Һ��ȫ���գ�����Һ����仯���Բ��ƣ������ձ�����ȡ20.00 mL������Һ����1.00mol/LNaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����16.00mLNaOH��Һ����ͼ�мг�װ���ԣ�
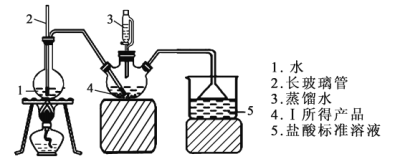
(4)����ƿ�з����Ļ�ѧ��Ӧ����ʽΪ____________________________��
(5)װ����2������Ϊ__________________________________________��
(6)��1.00mol/LNaOH����Һ�ζ���ʣ��HClʱ��ѡָʾ��Ϊ_________������ĸ����
a��ʯ����Һ b����̪��Һ c������
(7)��Ʒ����Ϊ____________________��
(8)����ʵ���������ʹ������(Sr3N2)�ⶨ���ƫ�ߵ���_________������ĸ����
a���ζ�ʱδ��NaOH����Һ��ϴ�ζ���
b������ʱ���ζ�ǰƽ�ӣ��ζ�����
c��ҡ����ƿʱ��Һ�彦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������³������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ2��.��ͼ��ʾΪ�������ؾ����һ����������������С���ظ���Ԫ����������˵������ȷ���ǣ�������
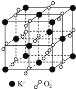
A. �������صĻ�ѧʽΪKO2��ÿ����������4��K+��4��O2-
B. ������ÿ��K+��Χ��8��O2-��ÿ��O2-��Χ��8��K+
C. ��������ÿ��K+���������K+��8��
D. �����У�0������2��������Ŀ��Ϊ2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ѱ���Ϊ��������֮��ĵ����������ش��������⣺
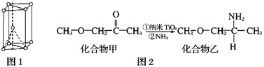
(1)�����Ѿ�����ͼ1��ʾ��Ϊ___________�ѻ�����ѻ���ʽ����
(2)����TiO2��һ��Ӧ�ù㷺�Ĵ����������һ��ʵ����ͼ2���������ҵķе����Ը��ڻ�����ף���Ҫԭ����________________��
(3)�������Ѿ�����������Ϊ��״�ۺ���ʽ�����ӣ��ṹ��ͼ3��ʾ���仯ѧʽΪ_______________��
(4)���ѿ���Ľṹ��ͼ4��ʾ��������λ�����������Ķ��ǣ���__________�������Ӱ�Χ����λ�����壻������λ���������������ģ���__________�������Ӱ�Χ�����ѿ���Ļ�ѧʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��Ԫ�����ڱ��е�ǰ�����ڣ��١���Ϊ��Ӧ��Ԫ�أ������ѡ����ʵ�Ԫ�ػش����⣺
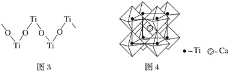
(1)����Ԫ��ԭ�ӵ���Χ�����Ų�������Ԫ�����ڱ��ɻ���Ϊ�������Ԫ��λ�����ڱ���__________����
(2)�ڡ�����Ԫ���γɵĻ�����Ŀռ乹��Ϊ__________��������ԭ�ӵ��ӻ��������Ϊ__________��
(3)д��Ԫ�آ��̬ԭ�ӵĵ����Ų�ʽ_______________________��
(4)�ۢܢݢ�����Ԫ�ص�һ�����ܵ���С�����˳��Ϊ______________(��Ԫ�ط���)��
(5)��ۢ��γɵ���ԭ�ӻ����ﻥΪ�ȵ�����ķ���Ϊ__________________��
(6)Ԫ�آ���CO���γɵ�X(CO)5�ͻ�����û����ﳣ���³�Һ̬���۵�Ϊ20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��жϸû����ᄃ������__________���壨������ͣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E��F����Ԫ�أ�����A��B��C��D��E�Ƕ�����Ԫ�أ�FΪ���ɽ�����ԭ��������������A��B����ͬһ���ڣ�B���������������ڲ��������3����C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ����ֻ�������ҡ�D��A��ԭ�Ӹ�����3��2�γ����ӻ��������E�ǵؿ��к�����ߵĽ���Ԫ�أ�F�������������������Ľ���������������Ϣ�ش��������⣺
��1��Aԭ�������ĵ����Ų�ͼ_______
��2��BԪ�������ڱ��е�λ����______________
��3���������ҵĻ�ѧ��������________________
��4����������ĵ���ʽ��____________
��5��F�ĵ����Ų�ʽ��__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(H3BO3)��һ��Ƭ��״�ṹ��ɫ���壬���ڵ�H3BO3����ͨ���������(����ͼ)�������й�˵����ȷ����
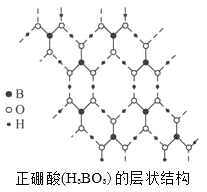
A. ��H3BO3�����и�ԭ�������ȫ������8�����ȶ��ṹ
B. H3BO3���ӵ��ȶ���������й�
C. 1mol H3BO3�ľ�������3mol���Թ��ۼ�
D. 1mol H3BO3�ľ�������3mol���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com