
 ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | �ζ����ʱ��NaOH��Һ����������mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
| c(��)��V(��) |
| V(��) |
| V(��)��c(��) |
| V(����) |
| 22.62mL+22.72mL+22.82mL |
| 3 |
| 0.10mol/L��22.72mL |
| 20.00mL |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
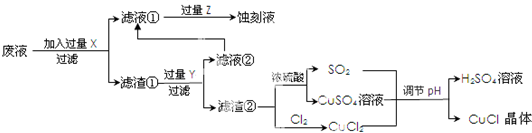
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | ����ȫȼ�պ�IJ�����n��CO2����n��H2O��=2��1 ��28��Mr��A����60 �۲���ʹ������Ȼ�̼��Һ��ɫ ��һ�ȴ���ֻ��һ�ֽṹ ��̼̼֮�䶼�ǵ��� |
| B | �ٱ���������ͨ������³���̬ �ڴ���ͬ���칹�� �۶������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� | ����NaOH ��Һ������� | 0.1000mol/L��������/mL[ѧ+ | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.28 | 26.28 |
| �ڶ��� | 25.00 | 1.55 | 30.30 | 28.75 |
| ������ | 25.00 | 0.20 | 26.42 | 26.22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
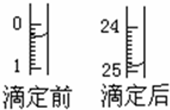
| 1 | 2 | 3 | |
| 0.25����Ʒ�������������Һ�������ml�� | 24.35 | 24.05 | 23.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�
ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�| �ζ���� | ����Һ�����mL�� | �����������Һ�������mL�� | ||
| �ζ�ǰ | �ζ��� | ���ĵ���� | ||
| 1 | 25.00 | 0.50 | 24.95 | 24.45 |
| 2 | 25.00 | |||
| 3 | 25.00 | 6.00 | 30.55 | 24.55 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ij����W�м���ϡ���ᣬ��������ɫ��ζ������ͨ�����ʯ��ˮ | ʯ��ˮ����� | W������Na2CO3 |
| B | պ��Ũ��ˮ�IJ�����������ҺX | �а��̲��� | Xһ����Ũ���� |
| C | ǿ������ҺY�м���Ba��NO3��2��Һ�����ú��ټ���KSCN��Һ | ���а�ɫ������ ����Һ�ֱ�� | Y��һ������SO42-��Fe3+ |
| D | ������̬Ȳ��Zͨ��pH=a����ˮ�� | ��ˮ��ɫ����Һ��pH��ԼΪa | Z���巢����ȡ����Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ����� | ���� | ���� |
| A | ��ij��Һ�еμ������ữ���Ȼ�����Һ | ���ְ�ɫ���� | ����Һ��һ������SO42 |
| B | ���մ��С�մ���Һ�зֱ�������� | ��ð���� | -���߾��������ᷴӦ |
| C | ���ɵ��߶ȵ�ͭ˿���뵽ϡHNO3�� | ��Һ���� | Cu��ϡHNO3�����û���Ӧ |
| D | ��KI��FeCl3��Һ���Թ��л�Ϻ���CCl4�������� | �ϲ���Һ ���Ϻ�ɫ | �����ԣ�Fe3+��I2 |
| A��A | B��B | C��C | D��D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ܽ��������BaCl2��Һ������ |
| B���ܽ��������CaCl2��Һ������ |
| C���ܽ��������Ca��OH��2��Һ������ |
| D���ܽ����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NH4��2Fe��SO4��2��Һ��c��SO42-����c��NH4+����c��Fe2+����c��H+�� |
| B��NH4Cl��Һ��c��Cl-����c��NH4+����c��OH-����c��H+�� |
| C��Na2CO3��Һ��c��Na+��+c��H+��=c��CO32-��+c��HCO3-��+c��OH-�� |
| D��NaHCO3��Һ��c��Na+��+c��H+��+c��H2CO3��=c��OH-��+c��CO32-�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com