

 +O2 $��_{��}^{����}$
+O2 $��_{��}^{����}$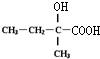 +H2O��
+H2O��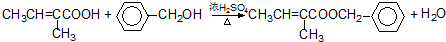
 ��
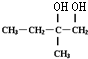
������ ����A����ˮ�ļӳɲ�����ж�AΪCH2=C��CH3��CH2CH3����Ӧ����ˮ�ⷴӦ��������BΪ ��B����������Ӧ�õ�C����DΪ
��B����������Ӧ�õ�C����DΪ ������C��D�ķ���ʽ�Ŀ��жϣ���Ӧ������ȥ��Ӧ����DΪCH3CH=C��CH3��COOH����Ӧ������±������ˮ�ⷴӦ����E�Ľṹ��ʽΪ
������C��D�ķ���ʽ�Ŀ��жϣ���Ӧ������ȥ��Ӧ����DΪCH3CH=C��CH3��COOH����Ӧ������±������ˮ�ⷴӦ����E�Ľṹ��ʽΪ ��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ
��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ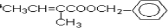 ���ݴ˽��
���ݴ˽��
��� �⣺����A����ˮ�ļӳɲ�����ж�AΪCH2=C��CH3��CH2CH3����Ӧ����ˮ�ⷴӦ��������BΪ ��B����������Ӧ�õ�C����DΪ
��B����������Ӧ�õ�C����DΪ ������C��D�ķ���ʽ�Ŀ��жϣ���Ӧ������ȥ��Ӧ����DΪCH3CH=C��CH3��COOH����Ӧ������±������ˮ�ⷴӦ����E�Ľṹ��ʽΪ
������C��D�ķ���ʽ�Ŀ��жϣ���Ӧ������ȥ��Ӧ����DΪCH3CH=C��CH3��COOH����Ӧ������±������ˮ�ⷴӦ����E�Ľṹ��ʽΪ ��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ
��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ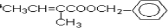 ��
��
��1��BΪ ������ʽΪ C5H12O2���ں˴Ź����������ܳ���6�ַ壬
������ʽΪ C5H12O2���ں˴Ź����������ܳ���6�ַ壬
�ʴ�Ϊ��C5H12O2��6��
��2��DΪCH3CH=C��CH3��COOH�����еĹ����������ǣ��Ȼ���̼̼˫����
�ʴ�Ϊ���Ȼ���̼̼˫����
��3�������ϳ�·��������ȡ����Ӧ���ǣ��ڢݢޣ�
�ʴ�Ϊ���ڢݢޣ�
��4����Ӧ�۵Ļ�ѧ����ʽΪ�� +O2 $��_{��}^{����}$
+O2 $��_{��}^{����}$ +H2O��
+H2O��
��Ӧ�Ļ�ѧ����ʽ��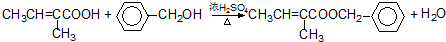 ��
��
�ʴ�Ϊ�� +O2 $��_{��}^{����}$
+O2 $��_{��}^{����}$ +H2O��
+H2O��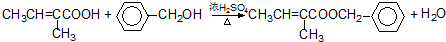 ��
��
��5��F��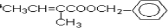 ���ж���ͬ���칹�壬������������ͬ���칹�壺�����ڷ����廯����Һ�����F��ͬ�Ĺ����ţ�����������̼̼˫�����ڱ�����������ȡ�������ұ����ϵ�һ�ȴ���ֻ�����֣�2����ͬ��ȡ�������ڶ�λ��������ȡ������̼ԭ����Ŀ��ͬ���Ҿ�����һ�ֹ����ţ�����һ������Ϊ��-CH=CHCH3���������Ϊ��-OOCCH2CH3��-CH2OOCCH3��-CH2CH2OOCH��-CH��CH3��OOCH��-COOCH2CH3��-CH2COOCH3������һ������Ϊ��-CH2CH=CH2���������Ҳ������6�֣�����һ������Ϊ
���ж���ͬ���칹�壬������������ͬ���칹�壺�����ڷ����廯����Һ�����F��ͬ�Ĺ����ţ�����������̼̼˫�����ڱ�����������ȡ�������ұ����ϵ�һ�ȴ���ֻ�����֣�2����ͬ��ȡ�������ڶ�λ��������ȡ������̼ԭ����Ŀ��ͬ���Ҿ�����һ�ֹ����ţ�����һ������Ϊ��-CH=CHCH3���������Ϊ��-OOCCH2CH3��-CH2OOCCH3��-CH2CH2OOCH��-CH��CH3��OOCH��-COOCH2CH3��-CH2COOCH3������һ������Ϊ��-CH2CH=CH2���������Ҳ������6�֣�����һ������Ϊ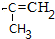 �ʹ���18�֣�����һ�ֽṹ��ʽΪ��
�ʹ���18�֣�����һ�ֽṹ��ʽΪ�� �ȣ�
�ȣ�
�ʴ�Ϊ��18�� ��
��
���� ���⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�ע�������÷���ʽ�����ƶϲ�ȡ���������Ͻ����ƶϣ����չ����ŵ������Լ������ŵ�ת��Ϊ������Ĺؼ���


 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʹpH��ֽ�ʺ�ɫ����Һ��Na+��NH4+��I-��NO3- | |
| B�� | ������pH=12����Һ��Na+��K+��SiO32-��NO3- | |
| C�� | c��Fe3+��=0.1mol•L-1����Һ��H+��Al3+��I-��SCN- | |
| D�� | ������������H2����Һ��K+��Mg2+��SO42-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
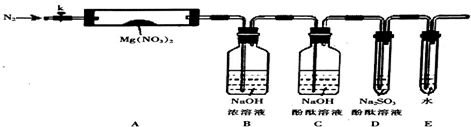
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2OH+CH3COOH $��_{��}^{Ũ����}$CH3COOCH2CH3+H2O | |
| B�� | CH2=CH2+HCl$\stackrel{����}{��}$CH3CH2Cl | |
| C�� | 2CH3CH2OH+O2$��_{��}^{����}$2CH3CHO+2H2O | |
| D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
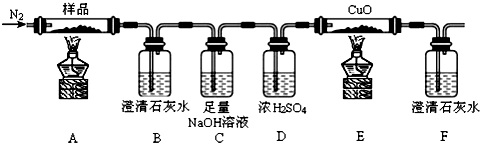
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ222 | |
| B�� | ������Ϊ86 | |
| C�� | ������Ϊ308 | |
| D�� | ��${\;}_{86}^{219}$Rn��${\;}_{86}^{220}$Rn��Ϊͬλ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Si����������ά | |
| B�� | �������ƿ�����DZͧ������ | |
| C�� | ˮ�������ݹ���ľ�ļ��ܷ��������ͻ� | |
| D�� | �����£����ۡ����ۿɴ�����Ũ���ᡢŨ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��A��=0.5 mol/��L•s�� | B�� | v��B��=0.005 mol/��L•s�� | ||
| C�� | v��C��=0.8 mol/��L•min�� | D�� | v��D��=1.0 mol/��L•min�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Na2SO3�Ʊ�����SO2��SO${\;}_{3}^{2-}$+2H+�TSO2��+H2O | |
| B�� | Na2SO3��Һʹ��̪�Լ���죺SO${\;}_{3}^{2-}$+H2O�TOH-+HSO${\;}_{3}^{-}$ | |
| C�� | ��Ba��NO3��2��Һ����HSO${\;}_{3}^{-}$��HSO${\;}_{3}^{-}$+Ba2+�TBaSO3��+H+ | |
| D�� | ��NaHSO3��Һ����H2S���壺2H2S+H++HSO${\;}_{3}^{-}$�T3S��+3H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com