
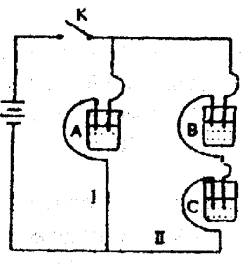
����ȥB����֪����ǿ��IA<IC������ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·����֪ͨ��A��B�����Һ�ĵ���ǿ������ǰͨ��A��Һ�ĵ���ǿ�ȵ���Դ�С��ϵΪ��IAB>IA����֪A��B��C�ֱ�ѡ��������Һ��0.1 mol•L��1���ᡢ0.1 mol•L��1���ᡢ0.1 mol•L��1 NaCl��Һ��0.1 mol•L��1���ᡢ0.1 mol•L��1 NaOH��Һ��0.1 mol•L��1��ˮ����25�棬A��ҺpH��7��
�ش��������⣺
��1��ָ��A��B��C������ʲô��Һ��
A�������������� B�������������� C��������������
��2������C��Һ�е����̪�Լ��ʺ�ɫ����C���������������� ��
��3����A��B��C�ֱ��Ե��������������ϣ���A��B��� ��A��C��� ��B��C��ϣ����Һ��ˮ�ĵ�����ɴ�С������˳��Ϊ�������� ������ţ���
| ��1��A��0.1 mol•L��1��� B��0.1
mol•L��1��ˮ�� C��0.1
mol•L��1�����ᡢ���ᡢ����������Һ���Ȼ�����Һ�е�һ�ֻ��־���
��2��0.1 mol•L��1����������Һ ��3���١��ڡ���
|
| ���⿼��ǿ������ʺ���Һ�����ԵĹ�ϵ����ѹ��ͬʱ����Һ�е�����������ƶ���������Խ�࣬��Һ�ĵ�����Խǿ��
��1����·���ӷ�ʽ��B��C������A��B��C�������ٸ���ʵ�������ɡ�I����I������֪A��B��C�ĵ�������������ȥB��IA<IC������֪C��һ��Ϊǿ����ʣ�A��Ϊ������ʣ���Bһ����������ʡ��˽��ۿ��ɡ�IAB>IA����һ��֤������Ŀ�и�����ǿ�������Һ�����֣�C��������һ�ֻ��־��ɡ�������ʹ����֣�����Ͱ�ˮ���ɡ�A��ҺpH��7����֪��AΪ���ᣬ��BΪ��ˮ�� ��2������C��Һ�е����̪�Լ��ʺ�ɫ����C���Ǽ��Ե�����������Һ�� ��3��A��B������ɴ���泥�A��C������ɴ����ƣ�B��C�Ļ��ҺΪ��ˮ������������Һ������NH4+��CH3COO����ˮ�е�H+��OH����������ѵ�����������NH3��H2O��CH3COOH���ٽ�ˮ�ĵ��룬ʹˮ�ĵ�����������Ի��Һ��ˮ�ĵ�����ɴ�С������˳���Ǣ١��ڡ��ۡ�
|


 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ͼ3-4
����ȥB����֪����ǿ�Ȣ�A������C������ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·����֪ͨ��A��B�����Һ�ĵ���ǿ������ǰͨ��A��Һ�ĵ���ǿ�ȵ���Դ�С��ϵΪ����sAB������A
��֪A��B��C�ֱ�ѡ��������Һ��
��0.1 mol��L-1���� ��0.1 mol��L-1���� ��0.1 mol��L-1NaCl��Һ ��0.1 mol��L-1���� ��0.1 mol��L-1 NaOH��Һ ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7
�ش��������⣺
(1)ָ��A��B��C��(�������)ʲô��Һ?
A____________��B____________��C____________��
(2)����C��Һ�е����̪�Լ��ʺ�ɫ����C��____________����A��B��C�ֱ��Ե��������������ϣ������������ϵĻ��Һ�У�ˮ�ĵ���̶����? ____________��(ѡ�A����B����C��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
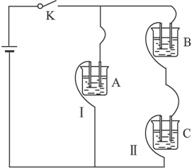
��֪A��B��C�ֱ�ѡ��������Һ��0.1 mol��L-1���ᡢ0.1 mol��L-1���ᡢ0.1 mol��L-1 NaCl��Һ��0.1 mol��L-1 NaOH��Һ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7��
����������⣺
��1��ָ��A��B��C�ǣ�������ǣ�ʲô��Һ��A.________��B.________��C.________��
��2������C��Һ�е����̪�Լ��ʺ�ɫ����C��________����A��B��C�ֱ��Ե��������������ϣ������������ϵĻ��Һ�У�ˮ�ĵ���̶����________��ѡ��A��B��C�ش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
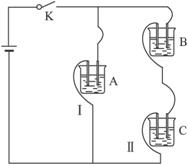
��֪A��B��C�ֱ�ѡ��������Һ��0.1 mol��L-1���ᡢ0.1 mol��L-1���ᡢ0.1 mol��L-1 NaCl��Һ��0.1 mol��L-1 NaOH��Һ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7��
����������⣺
��1��ָ��A��B��C�ǣ�������ǣ�ʲô��Һ��
A.______________��B.______________��C.______________��
��2������C��Һ�е����̪�Լ��ʺ�ɫ����C��________����A��B��C�ֱ��Ե��������������ϣ������________��ϵĻ��Һ�У�ˮ�ĵ���̶����ѡ��A��B��C�ش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C���ֵ������Һ�ֱ�װ�������ձ��У�����ʯī�缫������ͼ��ʾ��ʽ�ڵ�·�����ӡ��պϿ���K��ø�֧·����ǿ�Ȣ����֢���(���Т�����С)��

����ȥB����֪����ǿ�Ȣ�A������C������ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·����֪ͨ��A��B�����Һ�ĵ���ǿ������ǰͨ��A��Һ�ĵ���ǿ�ȵ���Դ�С��ϵΪ����sAB������A
��֪A��B��C�ֱ�ѡ��������Һ��
��0.1 mol��L-1���� ��0.1 mol��L-1���� ��0.1 mol��L-1NaCl��Һ ��0.1 mol��L-1���� ��0.1 mol��L-1 NaOH��Һ ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7
�ش��������⣺
(1)ָ��A��B��C��(�������)ʲô��Һ?
A____________��B____________��C____________��
(2)����C��Һ�е����̪�Լ��ʺ�ɫ����C��____________����A��B��C�ֱ��Ե��������������ϣ������������ϵĻ��Һ�У�ˮ�ĵ���̶����? ____________��(ѡ�A����B����C��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C���ֵ������Һ�ֱ�װ�������ձ��У�����ʯī�缫����ͼ��ʾ���ڵ�·�����ӿ���K��ø�֧·����ǿ��I��=I������I����С��������ȥB�����IA IC������ȥC������A��B����Һ��ϣ������Һ�ĵ���ǿ������ǰͨ��A�Ĵ�С��ϵΪIAB
IC������ȥC������A��B����Һ��ϣ������Һ�ĵ���ǿ������ǰͨ��A�Ĵ�С��ϵΪIAB 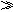 IA��
IA��
��֪A��B��C�ֱ�ѡ��������Һ��0.1mol?L-1���ᡢ0.1mol?L-1���ᡢ0.1mol?L-1NaCl��Һ��0.1mol?L-1���ᡢ0.1mol?L-1����������Һ��0.1mol?L-1��ˮ����25��ʱ��A��ҺpH<7���ش��������⣺
��1��ָ��A��B��C�ǣ�������ǣ�ʲô��Һ��д���ʻ�ѧʽ����
A________��B________��C________��
��2��A��B��C������Һ�ĵ���Ĵ�С��ϵΪ______���ɴ˿ɼ��������Һ�еĵ����С��______________�йء�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com