
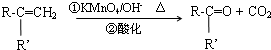
| 130-6��12-10��1 |
| 16 |
| ���� |
| �� |
| ���� |
| �� |

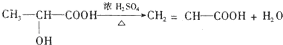
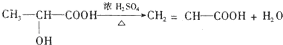



 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��R��R���ʾ����������ţ�
��R��R���ʾ����������ţ�
| ���� |
| �� |
| ���� |
| �� |






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ϳɾ۷���E��·�ߣ�
���ϳɾ۷���E��·�ߣ�






 �ṹ
�ṹ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�
��֪����R��R����ʾ����������ţ�
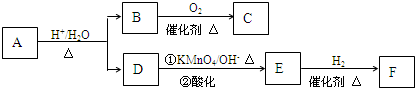
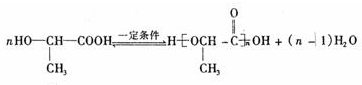
�л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5 mol A��ȫȼ��ֻ����3 mol CO2��2.5 mol H2O��A�ɷ�������ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ��������Ԫ��״������

��ش�
��1��1 mol B�������Ľ��������ò���22.4 L����״����H2��B�����������ŵ������� ��
B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ�� ��
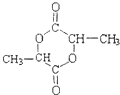
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������ �� ��
��3��F�ɷ����������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ�� ��
��F���Ƶ�ʹBr2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ�� ��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ�� ��
��4��A�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꼪��ʡ������и߶���ѧ���ڳ����Ի�ѧ�Ծ� ���ͣ������
��16�֣�
��֪�� ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�
�л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5 mol A��ȫȼ��ֻ����3 mol CO2��2.5 mol H2O��A�ɷ�������ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ��������Ԫ��״������
��ش�
��1��1 mol B�������Ľ��������ò���22.4 L����״����H2��B�����������ŵ������� ��
B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ�� ��
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������ �� ��
��3��F�ɷ����������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ�� ��
��F���Ƶ�ʹBr2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ�� ��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ�� ��
��4��A�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�켪��ʡ�߶���ѧ���ڳ����Ի�ѧ�Ծ� ���ͣ������
��16�֣�
��֪�� ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�
�л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5 mol A��ȫȼ��ֻ����3 mol CO2��2.5 mol H2O��A�ɷ�������ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ��������Ԫ��״������
��ش�
��1��1 mol B�������Ľ��������ò���22.4 L����״����H2��B�����������ŵ������� ��
B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ�� ��
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������ �� ��
��3��F�ɷ����������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ�� ��
��F���Ƶ�ʹBr2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ�� ��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ�� ��
��4��A�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com