
| ʱ��/��s�� | 0 | 20 | 40 | 60 | 80 | 100 |
| c��N2O4��/��mol/L�� | 0.20 | a | 0.10 | c | d | e |
| c��NO2��/��mol/L�� | 0.00 | 0.12 | b | 0.22 | 0.24 | 0.24 |
| ��C |
| ��t |
| C2(NO2) |
| C(N2O2) |
| ��C |
| ��t |
| 0.06mol/L |
| 20s |
| C2(NO2) |
| C(N2O2) |
| C2(NO2) |
| C(N2O2) |
| (0.24)2 |
| 0.08 |
| C2(NO2) |
| C(N2O2) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | �����ձ�ڵ���ͭ˿����װ������ˮ�Ҵ����Թ��� | ͭ˿��� | �Ҵ�����ȩ���л�ԭ�� |
| B | �����ɵ�AgI��Һ�е���KCl��Һ���� | ��������ɫ�������� | AgCl��AgI������ |
| C | Al������ϡHNO3�� | ������ | Al�����汻HNO3�����γ����ܵ�����Ĥ |
| D | �����Na2O2��ĩ����֬�������ˮ | ��֬����ȼ�� | Na2O2��CO2��ˮ�ķ�Ӧ�Ƿ��ȷ�Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��5��3 | B��3��5 |
| C��10��8 | D��8��8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
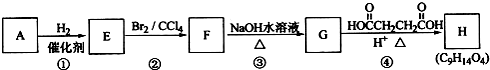
| H+ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
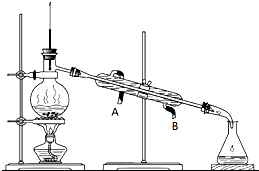 ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л��б�ͪ������������������������и����ʵ�����
ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л��б�ͪ������������������������и����ʵ�����| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е�/�� | 56.2 | 77.06 | 78.5 | 117.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��FeCl2��Һ��FeCl3�����ӹ�����ԭ���ۣ����� |
| B��KNO3��Һ��AgNO3�����ӹ���KCl ��Һ������ |
| C��NaCl��Һ��I2������CCl4����Һ |
| D��KNO3��Һ��NaCl��������������Ũ��Һ���¡����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com