
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
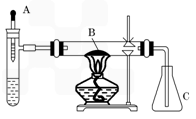
 ��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺
��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1������Ϊ��Ӧ���ܴ��ֽ�H2O2�������ǣ�д��ѧʽ��_____________��
��2���Թ��в�����������_____________��
��3��B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��4����ʵ�黹���Ը�������ҩƷ���������Թ��мӹ���___________����ͷ�ι�Ԥ������Һ��___________��
��5��������װ���⣬��ʵ��װ�õ�ȱ���ǻ�ȱ��___________װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
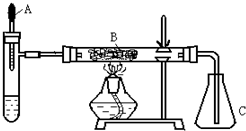
 ��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺
��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
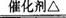
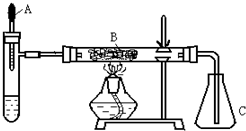
ͼ1-4
(1)����Ϊ��Ӧ���ܴ��ֽ�H2O2��������(д��ѧʽ)__________________��
(2)�Թ��в�����������_________��
(3)B�з�����Ӧ�Ļ�ѧ����ʽΪ__________________��
(4)��ʵ�黹���Ը�������ҩƷ���������Թ��мӹ���__________________����ͷ�ι�Ԥ������Һ��_________��
(5)������װ���⣬��ʵ��װ�õ�ȱ���ǻ�ȱ��_________װ�á�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com