
| A����̼�⻯����Ϊ2-��-1��3-���ϩ |
| B����̼�⻯�������嵥��1��1�ӳ�ʱ��������3�ֲ�ͬ�IJ��� |
| C����̼�⻯�������嵥��1��1�ӳ�ʱ��������2�ֲ�ͬ�IJ��� |
| D����̼�⻯���������������ӳ�ʱ�������������� |
| 0.72g |
| 18g/mol |
| 2.2g |
| 44g/mol |
| 5��2+2-8 |
| 2 |
| 32g |
| 160g/mol |
| 0.72g |
| 18g/mol |
| 2.2g |
| 44g/mol |
| 5��2+2-8 |
| 2 |
| 32g |
| 160g/mol |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ӳɷ�Ӧ |
| B��������Ӧ |
| C����Na��Ӧ |
| D����Na2CO3��Һ��Ӧ�ų�CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������15% | B������15% |
| C��С��15% | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��X��Y���ǽ���ʱ����Xһ����Y���� |
| B��Xһ�����ڽ����˳�����H��ǰ�� |
| C��X�ǽ���ʱ��Y�����ǽ���Ҳ�����Ƿǽ��� |
| D��X�Ƿǽ���ʱ��Y�����ǽ���Ҳ�����Ƿǽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���� | B���� | C���ڢ� | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� 2-�һ����� 2-�һ����� |
B��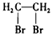 1��2-�������� 1��2-�������� |
C�� ����ױ� ����ױ� |
D�� 2-��-2-��ϩ 2-��-2-��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ������ʣ���ʵ��װ����ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���
ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ������ʣ���ʵ��װ����ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���| ���� | ���� | ���� | ��ϩ | ��ϩ | �� | �ױ� | ̼ |
| ����������%�� | 12 | 24 | 12 | 16 | 20 | 10 | 6 |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com