
����Ŀ����ش�
(1)������ Mg5Al3(OH)19(H2O)4 ������������ȼ���ϣ�����ʱ�����»�ѧ����ʽ�ֽ⣺2 Mg5Al3(OH)19(H2O)4 = 27H2O��+10MgO+3A12O3
��д���û���������ȼ��������_____
�������ӷ���ʽ��ʾ��ȥ��������� A12O3 ��ԭ��_____��
(2)þȼ�ղ����� CO2 ������û�ѧ����ʽ��ʾ������_____��
(3)�������������ɱ��������ܶ�ȡ�������е��������� 2Al+Fe2O3=Al2O3+2Fe���ҷ�Ӧ�ų��������ȣ���ұ��ѧ�ϳ�Ϊ���ȷ�Ӧ��ȡ�������ȷ�Ӧ���õĹ������������������ϡ���ᣬ�μ�KSCN ��Һ������������_____(������������������)˵�������������� Fe2O3��������_____��_____(�����ӷ���ʽ˵��)��
���𰸡���Ӧ���Ƚ����¶ȣ��������������������ˮ����ϡ�Ϳ��� Al2O3 + 2OH- = 2AlO2- + H2O 2Mg+CO2 == 2MgO+C ���� Fe2O3 6H =2Fe3 3H2O 2Fe3+ + Fe = 3Fe2+
��������
���ݽ���þ�����������仯��������ʷ�����𣻸������ӷ�Ӧ����ʽ��д����������
(1) �� 2Mg5Al3��OH��19��H2O��4![]() 27H2O��+10MgO+3Al2O3���ֽⷴӦ�����ȷ�Ӧ�������¶ȣ����ɵ�����þ�������������۵�ܸߵ���������ű������ֹȼ�գ��ʴ�Ϊ���ֽⷴӦ�����ȷ�Ӧ�������¶ȣ��������������������ˮ����ϡ�Ϳ�����
27H2O��+10MgO+3Al2O3���ֽⷴӦ�����ȷ�Ӧ�������¶ȣ����ɵ�����þ�������������۵�ܸߵ���������ű������ֹȼ�գ��ʴ�Ϊ���ֽⷴӦ�����ȷ�Ӧ�������¶ȣ��������������������ˮ����ϡ�Ϳ�����
������þ�Ǽ��������������ᣬ�����������������������ᡢ�����ڣ������������������ܽ����˳�ȥ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
(2) þȼ��ʱ����CO2��Ӧ������C��MgO���ʴ�Ϊ��2Mg+CO2 == 2MgO+C��
(3)�������ȷ�Ӧ�����ɵ�Fe���ʣ�δ��Ӧ��Fe2O3�����ᷴӦ���ɵ�Fe3+�������Fe����������ԭ��Ӧ�������ӷ���ʽΪ��Fe2O3 6H =2Fe3 3H2O 2Fe3+ + Fe = 3Fe2+�����Բ�����˵�����Ȳ����в�����Fe2O3���ʴ�Ϊ��������Fe2O3 6H =2Fe3 3H2O �� 2Fe3+ + Fe = 3Fe2+��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������
��1������������������أ��ٹ������ʵ��������������Ӵ�С�������Ӽ�ľ��롣�Թ��塢Һ�����ʶ��ԣ�����Ҫ��������________(��д��ţ���ͬ)���������������ʶ��ԣ�����Ҫ������____________������
��2����״���£����Ϊ11.2 L��CO2������________g��������ԭ�ӵ�������________����
��3������mgij���壬����˫ԭ�ӷ��ӹ��ɣ�����Ħ������ΪMg��mol��1���������ӵ�������NA��ʾ����
�ٸ���������ʵ���Ϊ________mol��
�ڸ������ڱ�״���µ����Ϊ________ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҽѧ�ϳ������Ը��������Һ�Ͳ�����Һ�ķ�Ӧ���ڲⶨѪ�Ƶĺ����ش��������⣺
(1)��ƽ�������ӷ���ʽ�����õ����ű�ʾ����ת�Ƶķ������Ŀ��
______+_____MnO4-+_____H2C2O4��_____CO2��+_____Mn2++____��
(2)�÷�Ӧ�еĻ�ԭ����______��
(3)��Ӧת����0.4mol���ӣ�������KMnO4�����ʵ���Ϊ______mol��
(4)��һ��������þ�����Ͻ�Ͷ��100 mLһ�����ʵ���Ũ�ȵ�HCl�У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5 mol��L-1NaOH��Һ�����������ɳ���������������NaOH��Һ�����ϵ����ͼ��
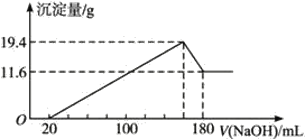
��ԭ�Ͻ�����������������___________��
����������ʵ���Ũ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ѽ��仯�����Ӧ����Խ��Խ�ܵ����ǵĹ�ע��
��1����̬��ԭ�Ӻ�������Ų�������ܼ��ķ�����_____________������ͬ���ڵ�Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����������ͬ����_____�֡�
��2���ѱȸ��ᡢ����Ӳ����һ�����˵Ľṹ���ϡ���Ӳ�ȱ������ԭ����________________________��
��3��TiCl4���Ȼ�����ȡ�ѵ��м���TiCl4��SiCl4�ڳ����¶���Һ�壬���ӽṹ��ͬ����������ķ�������SiCl4��TiCl4�Ļ����Ȼ�õ������______________���ѧʽ����
��4������Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ȵľۺϣ���ṹ����ͼ��ʾ��

����ɸ����ʵ�Ԫ���У��縺��������____________ ����Ԫ�����ƣ���
��M�У�̼ԭ�ӵ��ӻ���ʽ��________�֡�
��M�У�����_________�����ţ���
a. �м� b. �Ҽ� c.��λ�� d.��� e.���Ӽ�
��5�����ʯ(TiO2)�Ǻ��ѵ���Ҫ����֮һ�������ķ���ϵ�ṹ���侧���ṹ����������ͬλ�õ�ԭ����ͬ������ͼ��ʾ��

��4����A��B��C��D�У�������ԭ�ӵ���______________��
����A��B��C��ԭ������ֱ�ΪA (0,0,0)��B (0.69a,0.69a,c)��c (a, a,c)����D��ԭ������ΪD ��0.19a,_____��______�����������ļ���d=________________���ô���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)����VSEPR�ƶϷ��ӻ����ӵĿռ乹�͡�
PO![]() __________��CS2____________��AlBr3(���۷���)__________��
__________��CS2____________��AlBr3(���۷���)__________��
(2)�����ֻ��Է�Ӧ�м������ӣ����ǵ������о�����1��̼ԭ�Ӻ�3����ԭ�ӡ�������������������������ӵ����ģ�ͣ�д����Ӧ�Ļ�ѧʽ��
 ______��
______��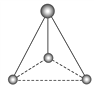 ______��
______��
(3)��Ҫ��д���ڶ����ڷǽ���Ԫ�ع��ɵ����Է��ӵĻ�ѧʽ��
ƽ�������η���________�������η���________���������η���________��
(4)Ϊ�˽��ͺ�Ԥ����ӵĿռ乹�ͣ���ѧ���ڹ�����������֪�ķ��ӿռ乹�͵Ļ����ϣ������һ��ʮ�ּ�����ģ�͡����۲���ӶԻ���ģ�͡�����ģ�Ͱѷ��ӷֳ����ࣺһ����____________________����һ����____________________��BF3��NF3�����ĸ�ԭ�ӵķ��ӣ�BF3������ԭ����________��NF3������ԭ����________��BF3���ӵ����幹����ƽ�������ζ�NF3���ӵ����幹���������ε�ԭ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(˫ѡ)���и��������У�����ԭ�ӵ��ӻ����������ͬ����(����)
A. NO��ClO B. SO![]() ��CO
��CO![]() C. NH��PH D. SO
C. NH��PH D. SO![]() ��SO
��SO![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ݸ�Ŀ���������վƵ����칤�գ������ỵ֮�ƣ��Կ����ա��� �����վƸ��ն��Ρ�����ֵ����Ҳ�����䷽��������������ʵ�ʵ�鷽��ԭ������ͬ����
A.���Ȼ�̼��ˮ
B.����غ��Ȼ���
C.�����������е�77 �棩�����ᣨ118 �棩�������ܣ�
D.ʳ��ˮ����ɳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E����Ԫ�ص�ԭ������������������Aԭ����������������������������ԭ��������ȣ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��s�����������p�����������������Dԭ��L������2�ԳɶԵ��ӣ�E�����Ӻ�����3�������M��3d�������ȫ��������ش��������⣺
(1)EԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ________��
(2)B��C��D����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ________(��Ԫ�ط���)����ԭ����________________________________________________________________________��
(3)DԪ�����Ԫ����ȣ��縺�ԣ�D________(�>����������<��)F�����б�������֤����һ��ʵ����______(��ѡ�����)��
A�������·�������ɫ��D���ʵ���ɫ�� B��������D���⻯����ҷ�Ӧ������D�ĵ���
C������D�γɵĻ�������DԪ�س�����̬ D���Ƚ���Ԫ�صĵ�������������ʱ�õ��ӵ���Ŀ
(4)��A��C��Ԫ�ؿ��γɻ�����CA5���еĻ�ѧ������Ϊ________��
(5)B2A4����Ҫ��ʯ�ͻ���ԭ�ϣ�B2A4�ĽṹʽΪ________��B2A4ͨ���ۺϷ�Ӧ��������һ���л��߷��ӻ������Ӧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڽ����˵������ȷ����
A.������۲�����B.������ͨ����Ĥ
C.�����ܲ��������ЧӦD.���岻�ȶ������ú����ײ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com