
����Ŀ����ͼ��ʾΪijЩ�л���֮����ת����ϵ������A��B���ڷ����廯�����B������FeCl3��Һ������ɫ��Ӧ��H�Ǻ���һ������ʯ�ͻ�����չˮƽ��־�����ʡ�
��ش��������⣺
��1��д�����л�����Ľṹ��ʽ��G____________��B____________��
��2��д�����з�Ӧ���ͣ���_____________����______________��
��3��д����Ӧ�١��ܵĻ�ѧ����ʽ����______________����____________��
��4����������3��������B��ͬ���칹�����Ŀ��________����
�ٺ����ڶ�ȡ�������ṹ����B����ͬ�����Ţ۲���FeCl3��Һ������ɫ��Ӧд����������һ��ͬ���칹��Ľṹ��ʽ_____________��
���𰸡�CH3COONa 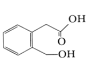 ˮ�� ��ȥ
ˮ�� ��ȥ ![]() ��2NaOH
��2NaOH![]() +CH3CH2OH��CH3COONa 2CH3CH2OH��O2
+CH3CH2OH��CH3COONa 2CH3CH2OH��O2![]() 2CH3CHO��2H2O 3
2CH3CHO��2H2O 3 ![]() ��
��![]() ��
��
��������
��H�Ǻ���һ������ʯ�ͻ�����չˮƽ��־�����ʿ�֪��HΪCH2=CH2����D������������������Ӧ��֪��D���ڴ�����D������ȥ��Ӧ���ɵõ���ϩ����DΪCH3CH2OH���Ҵ�������������Ӧ������CH3CHO����EΪCH3CHO����ȩ������������ͭ������Һ�б�����Ϊ�����ƣ���GΪCH3COONa����ϩ�����Ӿ۷�Ӧ���ɸ߾���![]() ��C���Ҵ���Ũ���������·���������Ӧ����F����F�ķ���ʽ��֪��CΪCH3COOH��FΪCH3COOCH2CH3��A����ˮ�����ữ�����Ҵ��������B��A��B���ڷ����廯�����B����ʹFeCl3��Һ����ɫ��˵��B�������ǻ��������Ȼ������ǻ�����B�����γ���Ԫ���������A�ķ���ʽ��֪��BΪ
��C���Ҵ���Ũ���������·���������Ӧ����F����F�ķ���ʽ��֪��CΪCH3COOH��FΪCH3COOCH2CH3��A����ˮ�����ữ�����Ҵ��������B��A��B���ڷ����廯�����B����ʹFeCl3��Һ����ɫ��˵��B�������ǻ��������Ȼ������ǻ�����B�����γ���Ԫ���������A�ķ���ʽ��֪��BΪ ��AΪ
��AΪ![]() ��
��
��1���ɷ�����֪��G�Ľṹ��ʽΪCH3COONa��B�Ľṹ��ʽΪ ���ʴ�Ϊ��CH3COONa��
���ʴ�Ϊ��CH3COONa�� ��
��
��2����Ӧ����![]() ������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�
������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�![]() ����Ӧ�����Ҵ���Ũ���������£����ȵ�170�淢����ȥ��Ӧ������ϩ��ˮ���ʴ�Ϊ��ˮ�ⷴӦ����ȥ��Ӧ��
����Ӧ�����Ҵ���Ũ���������£����ȵ�170�淢����ȥ��Ӧ������ϩ��ˮ���ʴ�Ϊ��ˮ�ⷴӦ����ȥ��Ӧ��
��3����Ӧ����![]() ������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�
������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�![]() ��Ӧ�Ļ�ѧ����ʽΪ
��Ӧ�Ļ�ѧ����ʽΪ![]() ��2NaOH
��2NaOH![]() +CH3CH2OH��CH3COONa ����Ӧ��Ϊ��ͭ�����������£��Ҵ�������������Ӧ������CH3CHO����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2
+CH3CH2OH��CH3COONa ����Ӧ��Ϊ��ͭ�����������£��Ҵ�������������Ӧ������CH3CHO����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O ���ʴ�Ϊ��
2CH3CHO��2H2O ���ʴ�Ϊ��![]() ��2NaOH
��2NaOH![]() +CH3CH2OH��CH3COONa ��2CH3CH2OH��O2
+CH3CH2OH��CH3COONa ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O ��
2CH3CHO��2H2O ��
��4��BΪ ��B��ͬ���칹����Ϻ����ڶ�ȡ�������ṹ����B����ͬ�����š�����FeCl3��Һ������ɫ��Ӧ��˵�������в����з��ǻ������д��ǻ����Ȼ�������������Ľṹ��ʽΪ
��B��ͬ���칹����Ϻ����ڶ�ȡ�������ṹ����B����ͬ�����š�����FeCl3��Һ������ɫ��Ӧ��˵�������в����з��ǻ������д��ǻ����Ȼ�������������Ľṹ��ʽΪ![]() ��
��![]() ��
�� ����3�֣��ʴ�Ϊ��
����3�֣��ʴ�Ϊ��![]() ��
��![]() ��
�� ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijԭ���װ����ͼ��ʾ������ܷ�ӦΪ2Ag��Cl2��2AgCl������˵����ȷ����

A. ������ӦΪAgCl ��e����Ag ��Cl��
B. �ŵ�ʱ������Ĥ�Ҳ���Һ���д�����ɫ��������
C. ����NaCl��Һ�������ᣬ�����ܷ�Ӧ��֮�ı�
D. ����·��ת��0.01 mol e��ʱ������Ĥ�����Һ��Լ����0.02 mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������Լ�ʵ��������ȫһ�µ��ǣ� ��
A | ������ͭ��Һ�м���һС������� | �к�ɫ�������� |
B | ��̼������Һ��ͨ����� | �а�ɫ�������� |
C | �����Ƶ���ˮ�ε���ɫʯ����ֽ�� | ��ֽ��� |
D | ����ɰֽ��ĥ�����������ھƾ��ƻ����ϼ��� | ���ۻ����������� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д�����з�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��1���Ҵ�����ϩ��____________����Ӧ���ͣ�____________��
��2���Ҵ���Ũ�����ᷴӦ��____________����Ӧ���ͣ�____________��
��3��������Ũ��ˮ��Ӧ��____________����Ӧ���ͣ�____________��
��4����ȩ��������Һ��Ӧ��____________����Ӧ���ͣ�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��![]() +CH2=CH-M
+CH2=CH-M![]() -M+HX��XΪ±ԭ�ӣ�MΪ������������ȡ������),���л���A�ϳ�G���㶹�أ��IJ������£�
-M+HX��XΪ±ԭ�ӣ�MΪ������������ȡ������),���л���A�ϳ�G���㶹�أ��IJ������£�
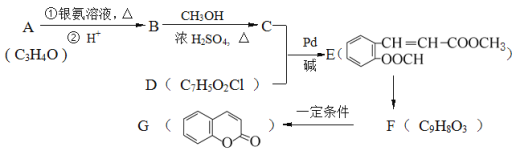
(1)д����ӦC+D��E�ķ�Ӧ���� ___________�� A�й���������Ϊ_________��
(2)д���ṹ��ʽ��B_____________ D______________��
(3)д����Ӧ����ʽ��E��F________��
(4)F�ж���ͬ���칹�壬д��ͬʱ������������������1��ͬ���칹��Ľṹ��ʽ��_______
�ٷ����г������⣬��������״�ṹ
�� �����������ֲ�ͬ��ѧ��������ԭ��
���ܷ���ˮ�ⷴӦ������������Ʒ�Ӧ
����������Cu(OH)2�����ʵ���֮��1��2��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ��ʳ�ΰ�װ��ǩ�ϵ����֡�
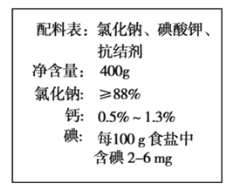
��1����ǩ�ϵ⺬���У�����ָ__________������ĸ��ţ���
A.��Ԫ�� B.�ⵥ�� C.������ D.������
��2�����ʵ�飺����![]()
![]() ��
��![]() ��Һ��
��Һ��
��ʵ������IJ����������ձ�������������Ͳ��_______����ͷ�ιܡ�
��������ƿ�м�������ˮ��ֱ��Һ���ƿ���̶�����![]() ʱ�����ý�ͷ�ιܵμ�����ˮ��Һ�����͵���̶���______________���Ǻ�ƿ�����������µߵ���ҡ�ȡ�
ʱ�����ý�ͷ�ιܵμ�����ˮ��Һ�����͵���̶���______________���Ǻ�ƿ�����������µߵ���ҡ�ȡ�
�����ƺõ���Һ__________����ܡ����ܡ������ڴ��������ƿ�С�
��3�����в����У��ᵼ��������ҺŨ��ƫ�͵���___________������ĸ��ţ���
A.ת����Һ������ƿ��δϴ���ձ��Ͳ�����
B.��Һʱ����ƿ������������ˮ
C.ҡ�Ⱥ���Һ���������ƿ�̶��ߣ���δ����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ�Һ�°�ĸ����Ĵ����������գ�ʹ������Ƚ�������Ϊ��°��Ƽ����������Ϊ��
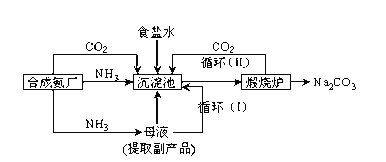
�ٳ������з�Ӧ�Ļ�ѧ����ʽ��______������¯�з����Ļ�ѧ����ʽ��__________
���ڳ����£���Na2CO3����Һ��ͨ��������CO2���о����������˾�����NaHCO3�������þ����ԭ����______________���ڱ��͵�ʳ��ˮ����ͨ�������İ�����ͨ��������CO2������NaHCO3�������������ڱ��͵�ʳ��ˮ����ͨ��������CO2����ͨ�백��������û�о���������ԭ��______��
�ۼ����Ʒ̼�������Ƿ����Ȼ��ƵIJ�������Ϊ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�����µļ��������ĵ����з��֣��³�����������������ϱ���������Ⱦ��Ҫ��Դ��������������ϣ����Ƕ�������������ж��л������һ���ж�����A��Ϊ�˲ⶨ�л���A�Ľṹ��������ʵ�飺
�ٽ�9.2g���л�����ȫȼ�գ����ɱ����15.68L��CO2��7.2gˮ��
���������Dzⶨ����Է�������������ͼһ��ʾ������ͼ��
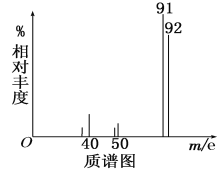
����ͼ��֪�÷��ӵ���Է���������________���л���A�ķ���ʽΪ_________��
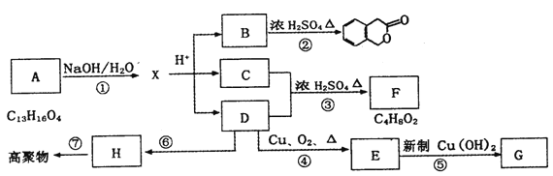
II. ��֪��A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����Aͨ���ۺϷ�Ӧ���ɸ߷��ӻ�����F��F�����ڹ�ҵ�ϳ����ϣ�����AΪ��Ҫԭ�Ϻϳ�G����AΪԭ�ϵĺϳ�·������ͼ��ʾ���ش��������⣺

(1)д���ڡ��� ����������Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��_____________________����Ӧ����__________��
��______________________����Ӧ����_________��
��_______________________����Ӧ����_________��
(2)д����C������������ͭ��Ӧ�Ļ�ѧ����ʽ��ʵ������_________________��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�������һ������������ǡ�
A.��pH��ֽ������ˮʪ����ij��Һ��pH
B.���к��ȵIJⶨʵ������![]() ����NaOH��
����NaOH��![]() ����HCl
����HCl
C.�ü�ʯ�������ﰱ��
D.ʹ��������ƽ����ʱ��ҩƷ������ƽ�����������������ƽ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com