
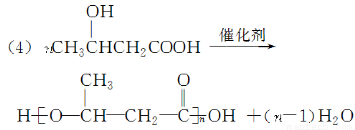
�ۣ�3���ǻ���������PHB������������ɽ������ϵȡ�PHB����3���ǻ�����[CH3CH��OH��CH2COOH]���Ӿۺ϶��ɡ��ϳɾۣ�3���ǻ���������;���ܶ���������һ��;���ĸ������١���ȾС��ԭ�������ʸ�����ϳ�·�����£�
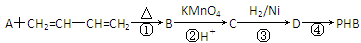
��֪�� 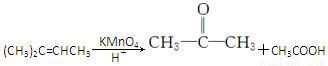
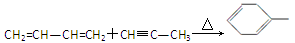
��ش��������⡣
��1��д��C�к��еĹ����ŵ����ƣ�____________��
��2������ת�����������ڼӳɷ�Ӧ����________������ţ���
��3����Ӧ��������ɫ��ѧ˼�루̼ԭ�ӵ���Ч������Ϊ100%������A�Ľṹ��ʽΪ__________________��
��4��д����Ӧ���Ļ�ѧ����ʽ��_____________________________________
_______________________________________________________________��
��5��д����C��Ϊͬ���칹�����ܷ���������Ӧ���Һ˴Ź����������������շ���л���Ľṹ��ʽ��____________________________________________��
��1���ʻ����Ȼ�����2���٢�����3��CH3��C��C��CH3
��5��OHC��CH2��O��CH2��CHO
�����������⿼���˹����������л���Ӧ���͵��жϡ�ͬ���칹��ͻ�ѧ����ʽ����д��֪ʶ�����ڿ��鿼�����л���ѧ֪ʶ�������������������֪���ղ���PHB����3���ǻ�����[CH3CH��OH��CH2COOH]���Ӿۺ϶������ó�DΪCH3CH��OH��CH2COOH����������Ϣ��֪A�Ľṹ��ʽΪCH3��C��C��CH3��BΪ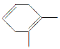 ��CΪ
��CΪ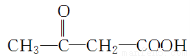 ��
��
��1��C�к��еĹ����ŵ��������ʻ����Ȼ���
��2�����������ڼӳɷ�Ӧ��
��3��A�Ľṹ��ʽΪCH3��C��C��CH3��
��4����Ӧ��Ϊ���۷�Ӧ����ѧ����ʽΪ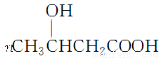
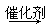
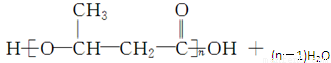
��5������Ҫ��Ľṹ��ʽΪOHC��CH2��O��CH2��CHO��


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��7�������Һ��ϰ���������棩 ���ͣ������
��ش��йصζ������е�������⡣
��1������֪Ũ�ȵ�����������Һ�ζ�δ֪Ũ�ȵ��������ζ�������ͼ��ʾ��
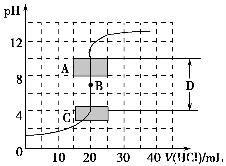
����ͼ��A��pH��Χʹ�õ�ָʾ����________��
C��pH��Χʹ�õ�ָʾ����________��
�����й��������к͵ζ������еIJ�������ȷ����________������ţ���
A���ü�ʽ�ζ�����ȡ��֪Ũ�ȵ��ռ���Һ
B���ζ��ܺ���ƿ�������ô�װҺ��ϴ
C���ζ�������ʼ��ע����ƿ����Һ��ɫ�仯
D����ƿ�еĴ���Һ������Ͳ��ȡ
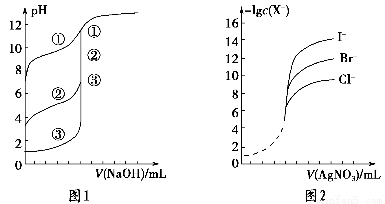
��2����ͼ1��ʾ����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ�Ũ����ͬ��3��һԪ������ͼ��ȷ��������ǿ����________����ͼ2��ʾ����ͬŨ�ȵ�����������Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ����ͼ��ȷ�����ȳ������� ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��4���ʽṹ��Ԫ����������ϰ���������棩 ���ͣ�ѡ����
����˵����ȷ���� ����������
A��ԭ�Ӻ˶��������Ӻ����ӹ��ɵ�
B��ͬλ�صĻ�ѧ���ʻ�����ͬ�����������ʲ�һ����ͬ
C������A��Ԫ��蘆�����ͬλ����137Cs��133Cs��4������
D����������ͬ����������ͬ��Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��2���û�ѧ����-���ʵ�����ϰ���������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ����������������� �� ����
A����״���£�22.4 L SO2��O2�Ļ�������к��е���ԭ����Ϊ2NA
B����״���£�22.4 L��ϩ�к��й��õ��ӶԵ���ĿΪ6NA
C���ܱ������У�3 mol H2��1 mol N2�ڸ��¡���ѹ�������������³�ַ�Ӧ�������ڵ������������Ϊ2NA
D��2.8 g��CO��2.8 g��N2������������Ϊ1.4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��15�л���ѧ����ѡ����ϰ���������棩 ���ͣ������
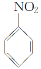
��Ʒ����Ϊ���а������л���F��һ��������ˮ�����ݼ�����ͨ�����кϳ�·���Ʊ���
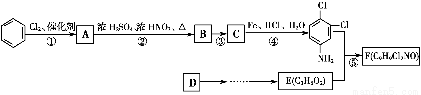
��֪����. 
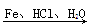
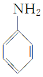 ���ʼ��ԣ�
���ʼ��ԣ�
��.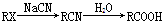
��.������ԭ��ȡ�����Ա������������ȡ������λ����һ��Ӱ����ʹ�µ�ȡ��������������λ����λ��ȡ�����У���CH3����NH2����X��±��ԭ�ӣ���ʹ�µ�ȡ�����������ļ�λ��ȡ�����У���COOH����NO2�ȡ�
��ش�
��1��F�Ľṹ��ʽΪ________��
��2����Ӧ���Ļ�ѧ����ʽΪ______________��
��3��������Ϊ����������������Ӧ˳��ߵ���Ҳ���Եõ�C��ʵ�����Dz��ġ�����ָ������֮��_______________________________________________��
��4����ɫ��ѧ��������ԭ����ԭ����ʵ�����ŷŻ����ŷš������̶�����Ϊ��ʵ����һ�������Ǹ�������_________________________________________________________��
��5����ԭ��DΪ��ϩ�������ṩ����Ϣ�����D��E��C3H6O2���ĺϳ�·�ߡ�
�ϳɷ�Ӧ����ͼ��ʾ������
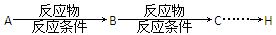
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��14���ʽṹ������ѡ����ϰ���������棩 ���ͣ������
�±�Ϊ���ڱ���һ���������еı�Ŵ�����Ӧ��Ԫ�ء�

����ա�
��1��д���ϱ���Ԫ��I�Ļ�̬ԭ�ӵĵ����Ų�ʽ�ͼ۲�����Ų�ͼ��________________________________________________________________________��
Ԫ��C��D��E��F�ĵ�һ�������ɴ�С��˳����________����Ԫ�ط��ű�ʾ����
��2��Ԫ��A�ֱ���C��D��E�γ����ij�����������Ӽס��Һͱ��������й���������ȷ����________��
A���ס��Һͱ����ӵĿռ乹�ͷֱ�Ϊ���������Ρ������Ρ�V��
B���ס��Һͱ�������������ԭ�Ӿ���ȡsp3���ӻ���ʽ
C�����ַ����м����ɴ�С��˳���DZ����ң���
D���ס��Һͱ����Ӿ�Ϊ�ɼ��Լ����ɵļ��Է���
��3����Ԫ��J��C��E���һ�ֻ�ѧʽΪJ��CE��5����λ�������������ʳ����³�Һ̬���۵�Ϊ��20.5 �棬�е�Ϊ103 �棬�����ڷǼ����ܼ����ݴ˿��жϣ�
���û�����ľ�������Ϊ________��
���û�����ľ����д��ڵ���������________��
A�����Ӽ� B�����Լ�
C���Ǽ��Լ� D�����»���
E����� F�����
�����ݹ��ۼ����ۺ͵ȵ��������۷�����CE��������������������Ŀ��Ϊ________��
��4���ڲⶨA��F�γɵĻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ����________________________________________________��
��5��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ�����������������Ԫ��G��Ԫ��B��ԭ����_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��13��ѧʵ���ۺ�Ӧ����ϰ���������棩 ���ͣ�ʵ����
���������μ����ֽ��Ҳ���ϸ��ӡ�ijѧϰС����Mg��NO3��2Ϊ�о���������ͨ��ʵ��̽�����ȷֽ�IJ������������4�ֲ��룺
�ף�Mg��NO2��2��NO2��O2
�ң�MgO��NO2��O2
����Mg3N2��O2
����MgO��NO2��N2
��1��ʵ��С���Ա�������϶����붡��������������______________________________��
�������ϵ�֪��2NO2��2NaOH=NaNO3��NaNO2��H2O
��Լס��ҡ����������������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
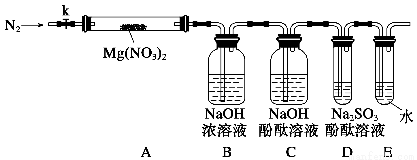
��2��ʵ�����
���������Ӻ�����������Լ�֮ǰ���ر�k����Ӳ�ʲ����ܣ�A�����۲쵽E�������������ų�������__________��
����ȡMg��NO3��2����3.7 g����A��������ǰͨ��N2������װ���ڵĿ�������Ŀ����________���ر�k���þƾ��Ƽ���ʱ����ȷ��������________��Ȼ��̶��ڹ��й��岿λ�¼��ȡ�
���۲쵽A���к���ɫ���������C��D��δ�����Ա仯��
������Ʒ��ȫ�ֽ���Aװ����ȴ�����¡����������ʣ����������Ϊ1.0 g��
��ȡ����ʣ��������Թ�������������ˮ��δ������������
��3��ʵ������������
������ʵ�������ʣ�����������������ɳ���ȷ�ϲ���______����ȷ�ġ�
������D��������������һλͬѧ��Ϊ����ȷ�Ϸֽ��������O2����Ϊ����O2��D�н�����������ԭ��Ӧ��______________����д��ѧ����ʽ������Һ��ɫ����ȥ��С�������϶��ֽ��������O2������δ����ԭ����_____________________________________��
��С�����ۺ��ɵĹ�ʶ������ʵ������Բ���������Ľ�װ�ý�һ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��12��ѧʵ�������ϰ���������棩 ���ͣ�ѡ����
������ͼ��ʾ���ܽ��������Ҫ�ӻ���Na2SO4��Na2Cr2O7�����еõ�Na2Cr2O7��������Ҫ������������������������������ ����������
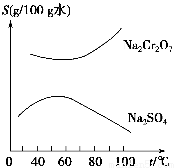
A�����½ᾧ B�����ȹ���
C�����½ᾧ D�������ᾧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-2��Դ�ۺ����á�����������ϰ���������棩 ���ͣ�ѡ����
������ɫ��ѧ�������У�����״̬�Ƿ�Ӧ���е�ԭ��ȫ��ת��Ϊ���ƵõIJ����ԭ��������Ϊ100%�����з�Ӧ������������ԭ�Ӿ�������ԭ����ǣ� ��
���û���Ӧ�������Ϸ�Ӧ�����ֽⷴӦ����ȡ����Ӧ�����ӳɷ�Ӧ������ȥ��Ӧ�����Ӿ۷�Ӧ�������۷�Ӧ
A���٢ڢ� B���ڢݢ� C���ߢ� D����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com