
��8�֣���1����֪25��ʱ������ʵ���ƽ�ⳣ����Ka��CH3COOH����1.8��10-5��Ka��HSCN����0.13��25��ʱ����20mL 0��10 mol��L��1 CH3COOH��Һ��20mL 0��10 mol��L��1HSCN��Һ�ֱ���20mL 0��10 mol��L��1NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼΪ��
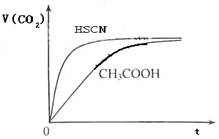
��Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ���� ��
��Ӧ��������������Һ�У�c��CH3COO���� c��SCN�������������������������
��2��25��ʱ����pH=1��H2SO4��Һa mL��pH=12��NaOH��Һb mL��Ϻ�������Һ��pH=3����a��b= ����Ӧ����Һ�и�����Ũ���ɴ�С��˳���� ��
��1��HSCN�����Ա�CH3COOHǿ������Һ��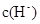 �ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ�
�� ��2��1 : 9��c��Na+��>c��SO
�ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ�
�� ��2��1 : 9��c��Na+��>c��SO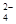 ��>c��H+��>c��OH����
��>c��H+��>c��OH����
����������1�����ݵ��볣����֪��HSCN�����Ա�CH3COOHǿ������Һ��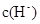 �ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ졣��Խ������Ӧ������Խ����ˮ�⡣����������Һ�ļ���ǿ��NaSCN��Һ�ļ��ԡ�����c��CH3COO����С��c��SCN������
�ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ졣��Խ������Ӧ������Խ����ˮ�⡣����������Һ�ļ���ǿ��NaSCN��Һ�ļ��ԡ�����c��CH3COO����С��c��SCN������
��2�������������������������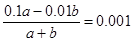 �����a��b=1 : 9�����ݵ���غ�c��Na+���� c��H+����2c��SO
�����a��b=1 : 9�����ݵ���غ�c��Na+���� c��H+����2c��SO ����c��OH������֪����Һ�и�����Ũ���ɴ�С��˳����c��Na+��>c��SO
����c��OH������֪����Һ�и�����Ũ���ɴ�С��˳����c��Na+��>c��SO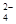 ��>c��H+��>c��OH������
��>c��H+��>c��OH������


 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡԪ��һ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��8�֣���1����֪25��ʱ������ʵ���ƽ�ⳣ����Ka��CH3COOH����1.8��10-5��Ka��HSCN����0.13��25��ʱ����20mL 0��10 mol��L��1 CH3COOH��Һ��20mL 0��10 mol��L��1HSCN��Һ�ֱ���20mL 0��10 mol��L��1NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼΪ��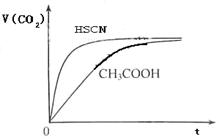
��Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ���� ��
��Ӧ��������������Һ�У�c��CH3COO���� c��SCN�������������������������
��2��25��ʱ����pH=1��H2SO4��Һa mL��pH=12��NaOH��Һb mL��Ϻ�������Һ��pH=3����a��b= ����Ӧ����Һ�и�����Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�ϲ��и�����ѧ�ڵ��п��Ի�ѧ�Ծ����������� ���ͣ������
��֪25��ʱ������ʵ���ƽ�ⳣ����
Ka��CH3COOH�� l.8 xl0��5,Ka��HSCN�� 0.13
��1����20mL��0.10mol/L CH3COOH��Һ��20mL��0.10mol/L��HSCN��Һ�ֱ���0.10mol/L��NaHCO3��Һ��Ӧ��ʵ���ò���CO2���������V����ʱ��t�Ĺ�ϵ��ͼ��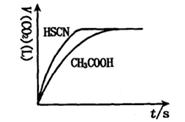
��Ӧ��ʼʱ��������Һ����CO2���������Բ�ͬ��ԭ���� ;
��Ӧ������������Һ��c��SCN����____ c��CH3COO�������>������=����<������
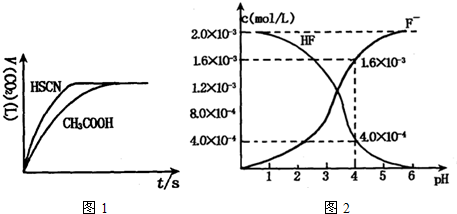
��2��2.0��l0��3mol/L�������ˮ��Һ�У�������ҺpH�����Ե���ʱ����仯�������ƽ����ϵ��c��F������c��HF������ҺpH�Ĺ�ϵ����ͼ��
��25��ʱ��HF����ƽ�ⳣ��Ϊ������ʽ��ֵ��Ka��HF��=
��3����������CaF2�ܶȻ�����Ϊ��Ksp= 1.5��10��10����4��0��10��3mol/L HF��Һ��4��0��l0��4 mol/L��CaCl2��Һ�������ϣ�������ҺpH =4�����Ե���ʱ��Һ����仯�����Է�����Ϻ��Ƿ��г������ɣ�____ ����С���û�С�����Ͳ�����ɣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�ϲ��и�����ѧ�ڵ��п��Ի�ѧ�Ծ��������棩 ���ͣ������
��֪25��ʱ������ʵ���ƽ�ⳣ����
Ka��CH3COOH�� l.8 xl0��5,Ka��HSCN�� 0.13
��1����20mL��0.10mol/L CH3COOH��Һ��20mL��0.10mol/L��HSCN��Һ�ֱ���0.10mol/L��NaHCO3��Һ��Ӧ��ʵ���ò���CO2���������V����ʱ��t�Ĺ�ϵ��ͼ��
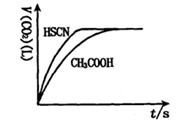
��Ӧ��ʼʱ��������Һ����CO2���������Բ�ͬ��ԭ���� ;
��Ӧ������������Һ��c��SCN����____ c��CH3COO�������>������=����<������
��2��2.0��l0��3mol/L�������ˮ��Һ�У�������ҺpH�����Ե���ʱ����仯�������ƽ����ϵ��c��F������c��HF������ҺpH�Ĺ�ϵ����ͼ��
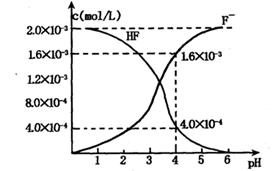
��25��ʱ��HF����ƽ�ⳣ��Ϊ������ʽ��ֵ��Ka��HF��=
��3����������CaF2�ܶȻ�����Ϊ��Ksp= 1.5��10��10����4��0��10��3mol/L HF��Һ��4��0��l0��4 mol/L��CaCl2��Һ�������ϣ�������ҺpH =4�����Ե���ʱ��Һ����仯�����Է�����Ϻ��Ƿ��г������ɣ�____ ����С���û�С�����Ͳ�����ɣ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com