
ЎҫМвДҝЎҝҪрКфҫ§МеөДФӯЧУ¶С»э·ҪКҪіЈУРТФПВЛДЦЦЈ¬ЗлИПХж№ЫІмДЈРН(јыНј)Ј¬»ШҙрПВБРОКМвЈә
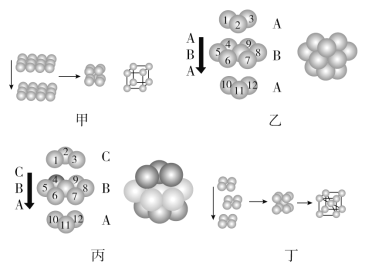
ЈЁ1Ј©ЛДЦЦ¶С»эДЈРНөД¶С»эГыіЖТАҙОКЗ________Ўў________Ўў________Ўў________ЎЈ
ЈЁ2Ј©јЧ¶С»э·ҪКҪЦРөДҝХјдАыУГВКОӘ________Ј¬Ц»УРҪрКф________ІЙУГХвЦЦ¶С»э·ҪКҪЎЈ
ЈЁ3Ј©ТТУлұыЦРБҪЦЦ¶С»э·ҪКҪЦРҪрКфФӯЧУөДЕдО»Кэ________(МоЎ°ПаН¬Ўұ»тЎ°І»ПаН¬Ўұ)Ј»ТТЦРөДҝХјдАыУГВКОӘ________ЎЈ
ЈЁ4Ј©ІЙИЎ¶ЎЦжѻэ·ҪКҪөДҪрКфНЁіЈУР________(ИОРҙИэЦЦҪрКфФӘЛШөД·ыәЕ)Ј¬Гҝёцҫ§°ыЦРЛщә¬УРөДФӯЧУКэОӘ________ЎЈ
Ўҫҙр°ёЎҝјтөҘБў·Ҫ¶С»э Бщ·ҪЧоГЬ¶С»э ГжРДБў·ҪЧоГЬ¶С»э МеРДБў·Ҫ¶С»э 52% Po(оЗ) ПаН¬ 74% KЎўNaЎўFe(әПАнјҙҝЙ) 2
ЎҫҪвОцЎҝ
ЈЁ1Ј©јтөҘБў·Ҫ¶С»эЈәҪ«·ЗГЬЦГІгөДҪрКфФӯЧУЙППВ¶ФЖлЈ¬РОіЙөДҫ§°ыКЗ1ёцБў·ҪМеЈ¬ФЪБў·ҪМеөДГҝёц¶ҘҪЗУР1ёцҪрКфФӯЧУЈ¬іЖОӘјтөҘБў·Ҫ¶С»эЈ»
Бщ·ҪЧоГЬ¶С»эУлГжРДБў·ҪЧоГЬ¶С»э¶јКЗЧоГЬ¶С»эЈ¬ЖдЦРБщ·ҪЧоГЬ¶С»эКЗТ»ЎўИэЎўОеЎӯёчІгЗтРДЦШәПЈ¬¶юЎўЛДЎўБщЎӯёчІгЗтРДЦШәПЈ»ГжРДБў·ҪЧоГЬ¶С»эКЗЛДЎўОеЎўБщЎӯІг·ЦұрәНТ»Ўў¶юЎўИэЎӯЗтРДЦШәПЈ¬Нј¶ЎөД¶С»э·ҪКҪКЗҪ«·ЗГЬЦГІгөДЙПІгҪрКфФӯЧУМоИлПВІгҪрКфФӯЧУРОіЙөД°јСЁЦРЈ¬ГҝІгҫщХХҙЛ¶С»эЈ¬РОіЙөДҫ§°ыКЗ1ёцБў·ҪМеЈ»
МеРДБў·Ҫ¶С»эЈәФЪБў·ҪМеөДГҝёц¶ҘҪЗУР1ёцФӯЧУЈ¬Бў·ҪМеөДЦРРДә¬УР1ёцҪрКфФӯЧУЈ»
ЈЁ2Ј©јтөҘБў·Ҫ¶С»эөДҝХјдАыУГВКЧоөНЈ¬ҝХјдАыУГВКОӘ52%Ј¬ІЙИЎХвЦЦ¶С»э·ҪКҪөДЦ»УРPoЈ»
ЈЁ3Ј©Бщ·ҪЧоГЬ¶С»эЎўЕдО»Кэ12ЎўҝХјдАыУГВК74%Ј»ГжРДБў·ҪЧоГЬ¶С»эЎўЕдО»Кэ12ЎўҝХјдАыУГВК74%
ЈЁ4Ј©МеРДБў·Ҫ¶С»эЎўЕдО»Кэ8ЎўГҝёцҫ§°ыЦРә¬УРҪрКфФӯЧУөДёцКэОӘЈә1+8ЎБ![]() =2ЎЈ
=2ЎЈ
ЈЁ1Ј©јЧөД¶С»э·ҪКҪКЗҪ«·ЗГЬЦГІгөДҪрКфФӯЧУЙППВ¶ФЖлЈ¬РОіЙөДҫ§°ыКЗ1ёцБў·ҪМеЈ¬ФЪБў·ҪМеөДГҝёц¶ҘҪЗУР1ёцҪрКфФӯЧУЈ¬іЖОӘјтөҘБў·Ҫ¶С»эЈ»ТТәНұы¶јКЗГЬЦГІгФӯЧУөД¶С»э·ҪКҪЈ¬ТТЦРЙПAІгәНПВAІгөД3ёцФӯЧУЧйіЙөДИэҪЗРО·ҪПтПаН¬Ј¬іЖОӘБщ·ҪЧоГЬ¶С»эЈ¬ұыЦРaІгәНCІгөД3ёцФӯЧУЧйіЙөДИэҪЗРО·ҪПтПа·ҙЈ¬іЖОӘГжРДБў·ҪЧоГЬ¶С»эЈ»Нј¶ЎөД¶С»э·ҪКҪКЗҪ«·ЗГЬЦГІгөДЙПІгҪрКфФӯЧУМоИлПВІгҪрКфФӯЧУРОіЙөД°јСЁЦРЈ¬ГҝІгҫщХХҙЛ¶С»эЈ¬РОіЙөДҫ§°ыКЗ1ёцБў·ҪМеЈ¬ФЪБў·ҪМеөДГҝёц¶ҘҪЗУР1ёцФӯЧУЈ¬Бў·ҪМеөДЦРРДә¬УР1ёцҪрКфФӯЧУЈ¬іЖОӘМеРДБў·Ҫ¶С»эЈ»
№Кҙр°ёОӘЈәјтөҘБў·Ҫ¶С»эЈ»Бщ·ҪЧоГЬ¶С»эЈ»ГжРДБў·ҪЧоГЬ¶С»эЈ»МеРДБў·Ҫ¶С»эЈ»
ЈЁ2Ј©јЧөД¶С»э·ҪКҪјтөҘБў·Ҫ¶С»эЈ¬јтөҘБў·Ҫ¶С»эөДҝХјдАыУГВКЧоөНЈ¬ҝХјдАыУГВКОӘ52%Ј¬ІЙИЎХвЦЦ¶С»э·ҪКҪөДЦ»УРPoЈ»
№Кҙр°ёОӘЈә52%Ј»PoЈ»
ЈЁ3Ј©ТТәНұыБҪЦЦ¶С»э·ҪКҪЦРЈ¬ҪрКфФӯЧУөДЕдО»КэҫщОӘ12Ј¬ЗТЖдҝХјдАыУГВКҫщОӘ74%Ј»
№Кҙр°ёОӘЈәПаН¬Ј»74%Ј»
ЈЁ4Ј©¶ЎКЗМеРДБў·Ҫ¶С»эЈ¬ІЙИЎХвЦЦ¶С»э·ҪКҪөДҪрКфУРKЎўNaЎўFeөИЈ¬УГҫщМҜ·ЁҝЙЗуөГГҝёцҫ§°ыЦРә¬УРҪрКфФӯЧУөДёцКэОӘЈә1+8ЎБ![]() =2Ј»
=2Ј»
№Кҙр°ёОӘЈәKЎўNaЎўFeЈЁәПАнјҙҝЙЈ©Ј»2ЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБР№ШУЪCЎўSiј°Жд»ҜәПОпҪб№№УлРФЦКөДВЫКцҙнОуөДКЗ
A.јьДЬ![]() Ўў
Ўў![]() Ј¬ТтҙЛC2H6ОИ¶ЁРФҙуУЪSi2H6
Ј¬ТтҙЛC2H6ОИ¶ЁРФҙуУЪSi2H6
B.Бў·ҪРНSiCКЗУлҪрёХКҜіЙјьЎўҪб№№ҫщПаЛЖөД№ІјЫҫ§МеЈ¬ТтҙЛҫЯУРәЬёЯөДУІ¶И
C.SiH4ЦРSiөД»ҜәПјЫОӘ+4Ј¬CH4ЦРCөД»ҜәПјЫОӘ-4Ј¬ТтҙЛSiH4»№ФӯРФРЎУЪCH4
D.SiФӯЧУјдДСРОіЙЛ«јь¶шCФӯЧУјдҝЙТФЈ¬КЗТтОӘSiөДФӯЧУ°лҫ¶ҙуУЪCЈ¬ДСРОіЙ![]() јь
јь
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝіЈОВПВЈ¬ПВБРУР№ШөзҪвЦКИЬТәөДЛө·ЁҙнОуөДКЗ
A.ПаН¬ЕЁ¶ИөД HCOONaәНNaFБҪИЬТәЈ¬З°ХЯөДpHҪПҙуЈ¬Фт ![]()
B.ПаН¬ЕЁ¶ИөДCH3COOHәНCH3COONaБҪИЬТәөИМе»э»мәПәуpHФјОӘ4.7Ј¬ФтИЬТәЦР![]()
C.FeSИЬУЪПЎБтЛбЈ¬¶шCuSІ»ИЬУЪПЎБтЛбЈ¬Фт![]()
D.ФЪ![]() ИЬТәЦРЈ¬
ИЬТәЦРЈ¬![]()
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝFeЎўCoЎўNiКЗИэЦЦЦШТӘөДҪрКфФӘЛШЎЈ»ШҙрПВБРОКМв:
(1)FeЎўCoЎўNiФЪЦЬЖЪұнЦРөДО»ЦГОӘ_________Ј¬»щМ¬FeФӯЧУөДөзЧУЕЕІјКҪОӘ__________ЎЈ
(2)CoOөДГжРДБў·Ҫҫ§°ыИзНјЛщКҫЎЈЙи°ў·ьјУөВВЮіЈКэөДЦөОӘNAЈ¬ФтCoOҫ§МеөДГЬ¶ИОӘ______g©qcm-3ЈәИэЦЦФӘЛШ¶юјЫСх»ҜОпөДҫ§°ыАаРНПаН¬Ј¬ЖдИЫөгУЙёЯөҪөНөДЛіРтОӘ_______ЎЈ

(3)FeЎўCoЎўNiДЬУлC12·ҙУҰЈ¬ЖдЦРCoәНОӘNiҫщЙъІъ¶юВИ»ҜОпЈ¬УЙҙЛНЖ¶ПFeCl3ЎўCoCl3әНCl2өДСх»ҜРФУЙЗҝөҪИхөДЛіРтОӘ____Ј¬Co(OH)3УлСОЛб·ҙУҰУР»ЖВМЙ«ЖшМеЙъіЙЈ¬Рҙіц·ҙУҰөДАлЧУ·ҪіМКҪЈә______ЎЈ
(4)95ЎжКұЈ¬Ҫ«NiЖ¬ҪюФЪІ»Н¬ЦКБҝ·ЦКэөДБтЛбЦРЈ¬ҫӯ4РЎКұёҜКҙәуөДЦКБҝЛрК§ЗйҝцИзНјЛщКҫЈ¬өұ![]() ҙуУЪ63%КұЈ¬Niұ»ёҜКҙөДЛЩВКЦрҪҘҪөөНөДҝЙДЬФӯТтОӘ_____ЎЈУЙУЪNiУлH2SO4·ҙУҰәЬВэЈ¬¶шУлПЎПхЛб·ҙУҰәЬҝмЈ¬№ӨТөЙПСЎУГH2SO4әНHNO3өД»мЛбУлNi·ҙУҰЦЖұёNiSO4ЎЈОӘБЛМбёЯІъОпөДҙҝ¶ИЈ¬ФЪБтЛбЦРМнјУHNO3өД·ҪКҪОӘ______(МоЎ°Т»ҙО№эБҝЎұ»тЎ°ЙЩБҝ¶аҙОЎұ)Ј¬ҙЛ·ЁЦЖұёNiSO4өД»ҜС§·ҪіМКҪОӘ_______ЎЈ
ҙуУЪ63%КұЈ¬Niұ»ёҜКҙөДЛЩВКЦрҪҘҪөөНөДҝЙДЬФӯТтОӘ_____ЎЈУЙУЪNiУлH2SO4·ҙУҰәЬВэЈ¬¶шУлПЎПхЛб·ҙУҰәЬҝмЈ¬№ӨТөЙПСЎУГH2SO4әНHNO3өД»мЛбУлNi·ҙУҰЦЖұёNiSO4ЎЈОӘБЛМбёЯІъОпөДҙҝ¶ИЈ¬ФЪБтЛбЦРМнјУHNO3өД·ҪКҪОӘ______(МоЎ°Т»ҙО№эБҝЎұ»тЎ°ЙЩБҝ¶аҙОЎұ)Ј¬ҙЛ·ЁЦЖұёNiSO4өД»ҜС§·ҪіМКҪОӘ_______ЎЈ

Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТСЦӘAКЗТ»ЦЦҪрКфЈ¬ЖдСжЙ«·ҙУҰіК»ЖЙ«Ј¬BИЬТәДЬК№·УМӘКФТәұдәмЈ»DЎўFПаУц»бІъЙъ°ЧСМЎЈAЎўBЎўCЎўDЎўEЎўFјдУРИзПВұд»Ҝ№ШПөЈә
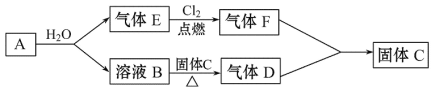
ЈЁ1Ј©AөДГыіЖКЗ___Ј»FөД»ҜС§КҪКЗ__ЎЈ
ЈЁ2Ј©BЎъD·ҙУҰөД»ҜС§·ҪіМКҪОӘ__ЎЈ
ЈЁ3Ј©FФЪҝХЖшЦРУцЛ®ХфЖшІъЙъ°ЧОнПЦПуЈ¬Хв°ЧОнКөјКЙПКЗ__ЎЈ
ЈЁ4Ј©ТСЦӘЈ¬ЖшМеDТІДЬУлCl2·ўЙъ·ҙУҰЈ¬КФРҙіцөұCl2ЧгБҝКұёГ·ҙУҰөД»ҜС§·ҪіМКҪЈ¬ІўУГЛ«ПЯЗЕ·ЁұкіцөзЧУЧӘТЖөДКэДҝ___ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіРЛИӨРЎЧйК№УГјЧНйИјБПөзіШ(ИзНјјЧЛщКҫ)ЧчОӘSO2ҙ«ёРЖч(ИзНјТТЛщКҫ)өДөзФҙЈ¬јмІвҝХЖшЦРSO2өДә¬БҝЎЈПВБРЛө·ЁҙнОуөДКЗ( )
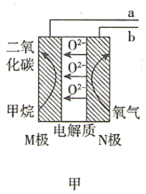
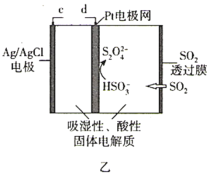
A.јЧНйИјБПөзіШMј«өДөзј«·ҙУҰКҪОӘCH4-8e-+4O2-=CO2+2H2O
B.јЧНйИјБПөзіШөДb¶ЛБ¬ҪУSO2ҙ«ёРЖчөДc¶Л
C.ұкЧјЧҙҝцПВЈ¬өұјЧНйИјБПөзіШөДNј«ПыәД2.24LөДO2КұҪшИлҙ«ёРЖчөДSO2ОӘ4.48L
D.ГҝЧӘТЖ1molөзЧУЈ¬ҙ«ёРЖчЦРAg/AgClөзј«ЦКБҝФцјУ35.5g
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРУР»ъОп·ЦЧУЦРЈ¬ЛщУРөДФӯЧУІ»ҝЙДЬҙҰУЪН¬Т»ЖҪГжөДКЗ ЈЁ Ј©
A.CH2=CHЎӘClB.CH2=CHЎӘCH=CH2
C.CH3ЎӘCH=CH2D.![]()
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ·ъБЧ»ТКҜКЗіЈјыөДёЖ·ъБЧЛбСОҝуОпЈ¬Жд»ҜС§КҪОӘCa5(PO4)3FЈ¬УЙ·ъБЧ»ТКҜЦЖИЎ»ЖБЧ(P4)өД»ҜС§КҪОӘ4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g)Ј¬Зл»ШҙрПВБРОКМвЈә
(1)»щМ¬CaФӯЧУөзЧУЛщХјҫЭЧоёЯДЬј¶өДөзЧУФЖВЦАӘНјОӘ________Ј¬»щМ¬PФӯЧУөДјЫөзЧУ№мөАұнҙпКҪОӘ________ЎЈ
(2)УЙ·ъБЧ»ТКҜЦЖИЎ»ЖБЧ(P4)ІъОпЦРКфУЪј«РФ·ЦЧУөД»ҜәПОпКЗ________Ј¬УлЖд»ҘОӘөИөзЧУМеөД·ЦЧУОӘ________ЎЈ
(3)»ЖБЧ(P4)ҫ§МеөДҝХјдҪб№№ИзНјјЧЛщКҫЈ¬PөДФУ»Ҝ№мөААаРНОӘ________Ј¬БЧөДТ»ЦЦБт»ҜОпP4S3өДҝХјдҪб№№ИзНјТТЛщКҫЈ¬ГҝёцSФӯЧУЦРә¬УР№ВөзЧУ¶ФөДКэДҝОӘ________ЎЈ


(4)SiO2өД·Рөг________(МоЎ°ҙуУЪЎұ»тЎ°РЎУЪЎұ)CO2өД·РөгЈ¬ЖдФӯТтОӘ________ЎЈ
(5)CaУлTiЎўOФӘЛШРОіЙөД»ҜәПОпөДҫ§°ыҪб№№ИзНјЛщКҫЈ¬ФтёГҫ§МеөД»ҜС§КҪОӘ________Ј¬Ифҫ§°ыГЬ¶ИОӘҰСgcm-3Ј¬°ў·ьјУөВВЮіЈКэөДЦөОӘNAЈ¬ФтёГҫ§°ыЦРБҪёцҫаАлЧоҪьөДOФӯЧУЦ®јдөДҫаАлОӘ________pm(УГә¬ҰСЎўNAөДұнҙпКҪұнКҫ)ЎЈ

Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝУыК№0.1molЎӨLЈӯ1өДNaHCO3ИЬТәЦРc(HЈ«)Ўўc(HCO3-)¶јјхЙЩ¶шc(CO32-)ФцҙуЈ¬Жд·Ҫ·ЁКЗ
A.НЁИл¶юСх»ҜМјЖшМеB.јУИлККБҝЗвСх»ҜДЖ№ММе
C.НЁИлВИ»ҜЗвЖшМеD.јУИлұҘәНКҜ»ТЛ®ИЬТә
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com