
��������ʮ�����ʣ���H2 ���� ��CuO ��CO2 ��H2SO4 ��Ba(OH)2 �ߺ��ɫ����������Һ�� �ఱˮ ��ϡ���� ��Al2(SO4)3
��1�������ʵķ������д����Ŀհ״���
| ����� | | ������ | | | ����� |
| ���ڸ�������� | �� | | ��� | �� | |
 H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��1��
��2��Ba(OH)2+2HNO3=Ba(NO3)2+2H2O����� �������� ������ ��Һ ���� ����� ���ڸ�������� �� �ۢ� ��� �� �ۢݢޢ�
��3��Al2(SO4)3= 2Al3++3SO42-�� 9.03��1022�� 0.6mol/L��
��4��Ba2++2OH-+CO2=BaCO3��+H2O��
��5��HNO3��1�U1��0.6mol�� Al+4H++NO3-=Al3++NO��+2H2O
���������������1��
�٢ڢۢܢݢߢ���д���ӷ�Ӧʽʱ�����ɷ֡��ޢⷴӦ��Ba SO4����������ֻ�Тޢ�������⡣����� �������� ������ ��Һ ���� ����� ���ڸ�������� �� �ۢ� ��� �� �ۢݢޢ�
Al2(SO4)3= 2Al3++3SO42- ;17.1g������ʵ���Ϊ0.05mol��SO42-��������Ϊ3��0.05mol��6.02��1023mol-1=9.03��1022��SO42-�����ʵ���Ũ��Ϊ3��0.05mol/250mL��10-3=0.6mol/L.
Ba2++2OH-+CO2=BaCO3��+H2O��
�����еĵ����ϼ۽��ͱ���ԭ������������������ѧ��ӦAl + 4HNO3 = Al(NO3)3 + NO�� + 2H2O�л�ԭ��Ϊ����������Ϊ���ᣬ����1/4���ᱻ��ԭ����1/4����Ϊ������������������ԭ��Ϊ1:1��5.4gAl=0.2molAl��0.2molAl������ΪAl3+ת�Ƶĵ�����Ϊ0.2mol��3=0.6mol���÷�Ӧ���ӷ�Ӧ����ʽΪAl+4H++NO3-=Al3++NO��+2H2O
���㣺���ʵķ��ࡢ���ӷ�Ӧ����ʽ��д��������ԭ��Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵���У���ȷ����
| A��CO��NO���Ǵ�����Ⱦ��ڿ����ж����ȶ����� |
| B��SiO2���������������NaOH��Һ��Ӧ |
| C����Һ�����������Һ�ı��������Ƿ�ɢ�����Ƿ����� |
D���ձ���й©������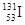 ԭ�ӣ����������� ԭ�ӣ�����������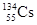 ����������2 ����������2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ͼ��ʾ�ش��������⣺
��1��������________��λ��1������________�ס�����ѧ�뼼�����о��ṹ�ߴ���1��100����Χ�ڲ��ϵ�������Ӧ�á�����________��ɢϵ�����Ӵ�Сһ����
��2����������С�����ֻ��ǧ���֮һ��������ô����ͼ�����ַ������ォ����������������������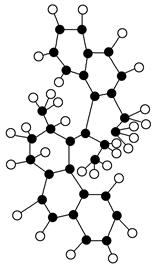
�ٸ�ͼ��������ӵ�____________ģ�͡�
�ڸ÷����к��е���ɻ���ԭ����____________Ԫ�ص�ԭ�ӣ������й���____________����ԭ�ӡ�
�����ײ�Ʒ��������������������������й���������Ʒ��˵���д������________��
a���ִ���ͥ�ձ�ʹ�õĵ������ǡ����ױ��䡱�����ĵ���������Ⱦ
b���ִ��̳���ĸߵ��·����ǡ������·���������ů����������Ⱦ
c��ר������ѧ�����õġ�Ӫ��ǿ��ţ�̡��ǡ�����ţ�̡�������ʹ����ǿ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʣ���Na2CO3 ��ͭ ���Ȼ��� ��CO2 ��NaHSO4 ��Ba(OH)2 �������������� �ఱˮ ��ϡ���� ��KI
��1�������ʵķ������д����Ŀհ״�(�����ʱ��)
| ����� | ����� | �� | �ǵ���� | ����� |
| ���ڸ��� ������ | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û��ϼۺ���������Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ�
��1���ӻ��ϼ۵ĽǶȿ���Ԥ�����ʵ����ʡ�
�� ������___________������ţ���ͬ����
������___________������ţ���ͬ����
A��ֻ�������� B��ֻ�л�ԭ�� C���������������л�ԭ��
�ڽ� ͨ������
ͨ������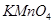 ��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ��������__________
��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ��������__________
A�� B��
B��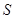 C��
C�� D��
D��
��2�������ʷ���ĽǶȿ����Ʋ����ʵ����ʡ�
����֪����ʯ��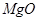 ��
�� ��
��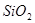 ��
��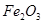 ��ɡ�����
��ɡ�����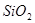 ����_______�����
����_______�����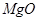 ��
��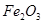 ����_________���������ԡ��������ԡ������ԡ�����
����_________���������ԡ��������ԡ������ԡ�����
����ȡһ������ʯ��������ʵ�飺
I���Ƚ������ڹ����������С����ˣ���������Ҫ�ɷ���_________��
II��������Һ�м���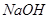 ��Һ�����������ˣ������е���Ҫ�ɷ���_________��
��Һ�����������ˣ������е���Ҫ�ɷ���_________��
����������������ʯ����ֱ�����ڹ�����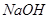 ��Һ�У���������������Ӧ�Ļ�ѧ����ʽ��_________________________��
��Һ�У���������������Ӧ�Ļ�ѧ����ʽ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���и������ʣ��������գ���
�� ���ʯ��ʯī��
��1H��2H��3H��
�� CH4��C10H22��
�������(CH3)2CHCH2CH3��
��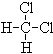 ��
��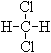 ��
��
�� CH3 (CH2) 3 CH3��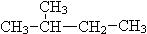
(1) ����ͬһ���ʵ��� ��
(2)��Ϊͬ���칹����� ��
(3)��Ϊͬϵ����� ��
(4) ��Ϊͬ����������� ��
(5) ��Ϊͬλ�ص��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij����ҩÿƬ��̼���500mg��������þ174mg����ҩ���кͶ��ٿ�������������Ϊ7��3�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����0��2 mol NaOH��0��1 mol Ba(OH)2����Һ�г����ȶ���ͨ��CO2���壬��ͨ������Ϊ8��96L(0�棬1��01��105 Pa)ʱ����ֹͣ������һ�����У���Һ�����ӵ����ʵ�����ͨ��CO2����������ϵͼ����ȷ����(������������ܽ��������ˮ��Ӧ) �� ��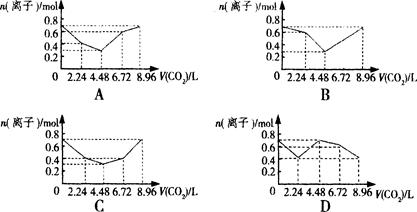
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl����Mg2+��Ba2+��CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г������ɣ�
��2���ڶ��ݼ�����NaOH��Һ���ռ�������0.05mol��
��3�������ݼ�����BaCl2��Һ�ø������4.3g������������ϴ�ӡ������������Ϊ2.33g��
��������ʵ�飬�����Ʋ���ȷ���ǣ� ��
| A��K+һ������ | B�������Һ��c(CO32��)Ϊ1 mol/L |
| C��Cl��һ������ | D��Ba2+һ�������ڣ�Mg2+���ܴ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com