
����ѧһһѡ�ޣ��л���ѧ��������11�֣�ij�л���A��C4H6O5���㷺����������ˮ���ڣ�����ƻ�������ѡ����ϡ�ɽ���Ϊ�࣬��һ�ֳ��õ�ʳƷ���Ӽ����û���������������ʣ�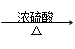 (i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6
(i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6 (ii) A + RCOOH(��ROH)
(ii) A + RCOOH(��ROH) ����ζ�IJ���
����ζ�IJ���
(iii) 1 mol A ��������1.5 mol����
(iv) �˴Ź������ױ���A��������5�ֲ�ͬ��ѧ��������ԭ��
��A��صķ�Ӧ��ͼ���£�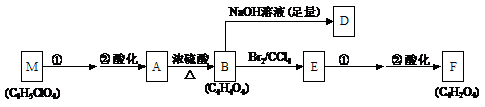
��1�����ݻ�����A�����ʣ���A�Ľṹ���������ж���_______��
��a���϶���̼̼˫�� ��b���������Ȼ�
��c���϶����ǻ� ��d���У�COOR������
��2��д��A��F�Ľṹ��ʽ��
A��___________________________�� F��___________________________��
��3��д��A��B��B��E�ķ�Ӧ���ͣ�A��B_______________�� B��E_______________��
��4��д�����з�Ӧ�ķ�Ӧ������
E��F�ڢٲ���Ӧ___________________��
��5���ڴ��������£�B���Ҷ����ɷ������۷�Ӧ�����ɵĸ߷��ӻ������������첣���֡�д���÷�Ӧ�Ļ�ѧ����ʽ��
___________________________________________________��
��6��д����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��
_________________________________________________��
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ѧһһѡ�����ʽṹ�����ʡ�
����ѧһһѡ�����ʽṹ�����ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧһһѡ�ޣ��л���ѧ��������11�֣�ij�л���A��C4H6O5���㷺����������ˮ���ڣ�����ƻ�������ѡ����ϡ�ɽ���Ϊ�࣬��һ�ֳ��õ�ʳƷ���Ӽ����û���������������ʣ�
![]() (i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6
(i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6
![]() (ii)A + RCOOH(��ROH)
(ii)A + RCOOH(��ROH) ����ζ�IJ���
(iii) 1 molA ��������1.5 mol����
(iv) �˴Ź������ױ���A��������5�ֲ�ͬ��ѧ��������ԭ��
��A��صķ�Ӧ��ͼ���£�
��1�����ݻ�����A�����ʣ���A�Ľṹ���������ж���_______��
��a���϶���̼̼˫�� ��b���������Ȼ�
��c���϶����ǻ� ��d���У�COOR������
��2��д��A��F�Ľṹ��ʽ��
A��___________________________�� F��___________________________��
��3��д��A��B��B��E�ķ�Ӧ���ͣ�A��B_______________�� B��E_______________��
��4��д�����з�Ӧ�ķ�Ӧ������
E��F�ڢٲ���Ӧ___________________��
��5���ڴ��������£�B���Ҷ����ɷ������۷�Ӧ�����ɵĸ߷��ӻ������������첣���֡�д���÷�Ӧ�Ļ�ѧ����ʽ��
___________________________________________________��
��6��д����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��
_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ׯ�Ӹ��и�����ѧ����ĩ����������ѧ�Ծ� ���ͣ������
����ѧһһѡ�ޣ��л���ѧ��������11�֣�ij�л���A��C4H6O5���㷺����������ˮ���ڣ�����ƻ�������ѡ����ϡ�ɽ���Ϊ�࣬��һ�ֳ��õ�ʳƷ���Ӽ����û���������������ʣ�
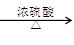 (i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6
(i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6
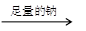 (ii)
A + RCOOH(��ROH)
(ii)
A + RCOOH(��ROH) ����ζ�IJ���
����ζ�IJ���
(iii) 1 mol A ��������1.5 mol����
(iv) �˴Ź������ױ���A��������5�ֲ�ͬ��ѧ��������ԭ��
��A��صķ�Ӧ��ͼ���£�
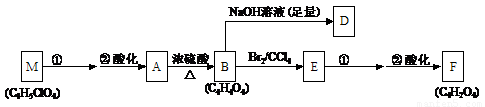
��1�����ݻ�����A�����ʣ���A�Ľṹ���������ж���_______��
��a���϶���̼̼˫�� ��b���������Ȼ�
��c���϶����ǻ� ��d���У�COOR������
��2��д��A��F�Ľṹ��ʽ��
A��___________________________�� F��___________________________��
��3��д��A��B��B��E�ķ�Ӧ���ͣ�A��B_______________�� B��E_______________��
��4��д�����з�Ӧ�ķ�Ӧ������
E��F�ڢٲ���Ӧ___________________��
��5���ڴ��������£�B���Ҷ����ɷ������۷�Ӧ�����ɵĸ߷��ӻ������������첣���֡�д���÷�Ӧ�Ļ�ѧ����ʽ��
___________________________________________________��
��6��д����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��
_________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com