

 ��ȩ�����л�ԭ�ԣ��ܱ�������Һ������������ͭ����Һ���������Գ���������Һ������������ͭ����Һ������ȩ����
��ȩ�����л�ԭ�ԣ��ܱ�������Һ������������ͭ����Һ���������Գ���������Һ������������ͭ����Һ������ȩ����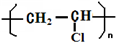 ��������Һ������������ͭ����Һ��
��������Һ������������ͭ����Һ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na2O+H2O�T2NaOH | ||||
| B��2F2+2H2O�T4HF+O2 | ||||
| C��Cl2+H2O�THCl+HClO | ||||
D��3Fe+4H2O
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ����SO32-���� |
| B��һ����Cl-���� |
| C��һ����CO32-���� |
| D������ȷ��HCO3-�����Ƿ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�ã�
���������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�ã�| a | b | c | |
| I | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ԭ����ǻ�ѧ�������һ���ش��ף�
ԭ����ǻ�ѧ�������һ���ش��ף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��A��B��C��D��E��FΪ����������Ԫ�أ�ԭ�Ӱ뾶��С��ϵΪA��D��C��B��F��E�� |
| ��A��D�γɵĻ����ﳣ����ΪҺ̬�� |
| ��BԪ��ԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�Ϊnsnnpn |
| ��FԪ��ԭ�ӵĺ���p����������s����������1�� |
| �ݵ�һ�����ܣ�F��E�� |
| ��G�Ļ�̬ԭ�Ӻ�����6��δ�ɶԵ��ӣ� |
| ��H���γɺ�ɫ����ש��ɫ����H2D�ͺ�ɫ��HD���ֻ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
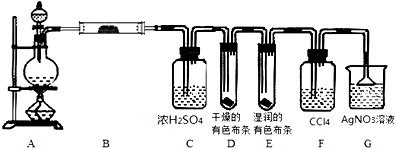
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol Cl2ͨ�뺬1molFeBr2����Һ�� Cl2+Fe2++2Br-=Fe3++Br2+2Cl- | ||
B��������NaHSO4�ӵ�������Ba��OH��2��Һ�� H++S
| ||
C��������ʯ��ˮ�ӵ�������NaHCO3��Һ�� Ca2++OH-+HC
| ||
| D������ͭ�����ᷴӦ O2-+2H+�TH2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com