
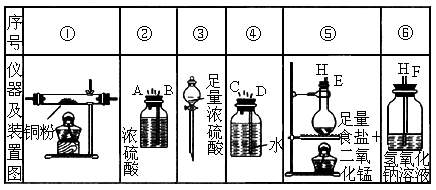
ʵ������������������ҩƷ����ȡ��������ˮ�Ȼ�ͭ��

ͼ��A��B��C��D��E��F�����߲��ֱ�ʾ�����ܽӿڣ��ӿڵ��������쳤�Ȳ���δ����������Ҫ����д���и�С��հס�
��1������������������������ʱ������������װ�õ���ȷ����˳���ǣ����װ�õ���ţ��� ���ӣ� ���ӣ� ���ӣ� ���ӣ� ���ӣ� ����
��2��ʵ�鿪ʼʱ��Ӧ���ȼ���װ�õ�____________��ʵ�����ʱ��Ӧ��Ϩ��_________
���ľƾ��ơ�
��3������ַ�Ӧ��װ�âٵIJ�������ʣ�����__________ɫ����ȴ���ƵõIJ�����ɱ�����Һ����Һ��__________ɫ��
��4��װ�âܵ�������________________________________________________________��
װ�â��е�������___________ _____________________________________��
������Ӧ�����ӷ���ʽ��________________ _________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���̲���ȫ��������л�ѧ��2�������ޣ�����³�ư�α�棩 ³�ư�α�� ���ͣ�022
ʵ������������ҩƷ����������ȡ��������ˮ�Ȼ�ͭ��

ͼ��A��B��C��D��E��F�����߲��ֱ�ʾ�����ܽӿڣ��ӿڵ��������쳤�Ȳ���δ����������Ҫ����д���и�С��հף�
(1)����������������������ʱ������������װ�õ���ȷ����˳����(���װ�õ����)(����)��(����)��(����)��(����)��(����)��(����)�����У������װ������ʱ�������ܽӿ�(��װ������ĸ��ʾ)Ӧ��________��________��
(2)װ�âڵ�������________��װ�âܵ�������________��װ�â��з�����Ӧ�Ļ�ѧ����ʽ��________��
(3)ʵ�鿪ʼʱ��Ӧ���ȼ���װ�õ�________��ʵ�����ʱ��Ӧ��Ϩ��________���ľƾ��ƣ�
(4)��װ�âݵ��ձ��У�������Ӧ�Ļ�ѧ����ʽΪ
________________________________________��
(5)����ַ�Ӧ��װ�âٵIJ�������ʣ�����________ɫ����ȴ���ƵõIJ�����ɱ�����Һ����Һ��________ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
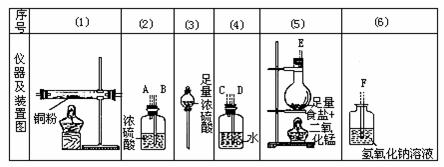
ʵ������������ҩƷ����������ȡ��������ˮ�Ȼ�ͭ��
ͼ��A��B��C��D��E��F�����߲��ֱ�ʾ�����ܽӿڣ��ӿڵ��������쳤�Ȳ���δ����������Ҫ����д���и�С��հף�
(1)����������������������ʱ������������װ�õ���ȷ����˳����(���װ�õ����)(����)��(����)��(����)��(����)��(����)��(����)�����У������װ������ʱ�������ܽӿ�(��װ������ĸ��ʾ)Ӧ��________��______��
(2)װ�âڵ�������______��װ�âܵ�������______��װ�â��з�����Ӧ�Ļ�ѧ����ʽ��__________��
(3)ʵ�鿪ʼʱ��Ӧ���ȼ���װ�õ�______��ʵ�����ʱ��Ӧ��Ϩ��______���ľƾ��ƣ�
(4)��װ�âݵ��ձ��У�������Ӧ�Ļ�ѧ����ʽΪ______��
(5)����ַ�Ӧ��װ�âٵIJ�������ʣ�����________ɫ����ȴ���ƵõIJ�����ɱ�����Ҵ����Һ��______ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08����һ����ģ����17�֣�ʵ������������װ�ã��̶�װ�á��������������ԣ������йذ�����ȡ��ʵ��̽�������ù����Լ��У�NH4Cl��NaCl��(NH4)2SO4��Ca(OH)2��CaO��NaOH��Һ���Լ��У�Ũ��ˮ��Ũ���ᡣ
 ������и��⣺
������и��⣺
��1�� ����װ�â���ȡNH3���䷴Ӧ�Ļ�ѧ����ʽΪ___________________________��Ҫ�ⶨ���ɵ�NH3�������������ѡ���װ����________________����װ����ţ���������ʢ�Լ�Ӧ���е������ǣ�___________________________________________
_______________________________________��
��2�� ����װ�â���ȡ���ռ������NH3����ƿ�ڵ��Լ�Ӧ��_____________����Һ©���е��Լ�Ӧ��______________���ռ�װ��Ӧѡ��________________����װ����ţ���֤���������ռ����IJ����ǣ�____________________________________________��
��3�� �������и����Լ���ϣ�����������ͬ��������ȡ�����ĶԱ�ʵ�飬��������������mL������״���£����±���
| 5.4g NH4Cl(s) | 5.4g (NH4)2SO4(s) |
6.0g Ca(OH)2(s,����) | ��1344 | ��1364 |
6.0g NaOH(s,����) | ��1568 | ��1559 |
6.0g CaO(s,����) | ��1753 | ��1792 |
�ӱ������ݷ�����ʵ������ȡ�����IJ�����ߵ���_____________������ţ�����ԭ����__________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������������������ҩƷ����ȡ��������ˮ�Ȼ�ͭ��ͼ��A��B��C��D��E��F�����߲��ֱ�ʾ�����ܽӿڣ��ӿڵ��������쳤�Ȳ���δ����������Ҫ����д���и��հף�
��1������������������������ʱ������������װ�õ���ȷ����˳���ǣ���д��װ�õ���ţ�_____��____��_ __��_ __��______��______�����У�2���루4������ʱ�������ܽӿڣ���װ������ĸ��ʾ��Ӧ��______��____��
��2������ַ�Ӧ��װ�ã�1���IJ�������ʣ�����_____ɫ����ȴ���ƵõIJ�����ɱ�����Һ����Һ��______ɫ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com