
����Ŀ��
��1��ij��̬�����ﻯѧʽΪRO2���ڱ�״���£�1.28 g������������Ϊ448 mL������������Ħ������Ϊ____________��R�����ԭ������Ϊ____________��
��2����֪CO��CO2�Ļ�����������Ϊ15 g���ڱ�״�������Ϊ8.8 L�������֪�û�������к�CO________ g������CO2�ڱ�״�������Ϊ____________ L��
��3����4 g NaOH����____________gˮ�У�����ʹÿ100��ˮ����������1��Na����
��4��ͬ��ͬѹ��SO2 �� He�ܶ�֮��Ϊ ����������ȣ�������������֮��____________
���𰸡�
��1��64g��mol��1 32
��2��4��5.6��
��3��180
��4��16��1��1��16
��������
�����������1������£�1.28g������������Ϊ448mL�������ʵ���Ϊ![]() =0.02mol���ʸ��������Ħ������=
=0.02mol���ʸ��������Ħ������=![]() =64g/mol��R�����ԭ������Ϊ64-32=32���ʴ�Ϊ��64g/mol��32��
=64g/mol��R�����ԭ������Ϊ64-32=32���ʴ�Ϊ��64g/mol��32��
��2�������������Ϊ8.96L�������ʵ���Ϊ![]() =0.393mol������������CO�����ʵ���Ϊxmol��CO2�����ʵ���Ϊymol����28x+44y=15��x+y=0.393�����x=0.15mol��y=0.25mol������m(CO)=0.15mol��28g/mol=4.2g�������0.25mol������̼���Ϊ22.4L/mol��0.1mol=5.6L���ʴ�Ϊ��4.2��5.6��
=0.393mol������������CO�����ʵ���Ϊxmol��CO2�����ʵ���Ϊymol����28x+44y=15��x+y=0.393�����x=0.15mol��y=0.25mol������m(CO)=0.15mol��28g/mol=4.2g�������0.25mol������̼���Ϊ22.4L/mol��0.1mol=5.6L���ʴ�Ϊ��4.2��5.6��
��3��NaOH�����ʵ���Ϊ![]() =0.1mol����ˮ�����ʵ���Ϊx����ÿ100��H2O��������һ��Na+����
=0.1mol����ˮ�����ʵ���Ϊx����ÿ100��H2O��������һ��Na+����![]() ��
��![]() �����x=10mol����ˮ������Ϊ10mol��18g/mol=180g����4g NaOH����180��ˮ�У�����ʹÿ100��H2O��������һ��Na+��
�����x=10mol����ˮ������Ϊ10mol��18g/mol=180g����4g NaOH����180��ˮ�У�����ʹÿ100��H2O��������һ��Na+��
��4����ͬ�����£��ܶ�֮�ȵ���Ħ������֮�ȣ���SO2�뺤�����ܶ�֮��=64g/mol��4g/mol=16��1����ͬ�����£�������ͬ���������֮����Ħ�������ɷ��ȣ�����������������=4g/mol��64g/mol=1��16���ʴ�Ϊ��16��1��1��16��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��1��ѡ����������ɱ�ʵ��������������У�������ƽ����ȷ��0.1g����ҩ�ס��ձ�����������________��________�Լ�����������Ƭ��ֽ��
��2�����㣮���Ƹ���Һ��ȡNaCl����_________g��
��3������������ƽ��ƽ֮��Ӧ����ƽ���������ij��λ�ã�����ͼ1����һ�����߱���������Ե������λ�ã�

������������NaCl����Ӧ������ƽ��________��������������������������
��������ϣ���ҩƷ�����ձ��У�
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����__________��
��5��ת�ơ�ϴ�ӣ���ת��ʱӦʹ��________��������Ҫϴ���ձ�2��3����Ϊ��________��
��6�����ݣ�ҡ�ȣ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�������ñ�ǩ��ע�����Ƶ�ʱ�䡢________��________��
��8�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ2��ʾ��������Һ��Ũ�Ȼ�________������ƫ��������ƫ����������Ӱ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������п(ZnO)��������(GaN)�����Ͷ��������ɵ����ײ��������ÿɼ���ֽ�ˮ������������������
��1��Zn2+��̬��������Ų�ʽΪ
��2����CNO����Ϊ�ȵ�����ķ��ӡ����ӻ�ѧʽ�ֱ�Ϊ �� (��дһ��)
��3��ZnO������������ܸ�ǿ����Һ��Ӧ����[Zn(OH)4]2-�������ǿռ乹�ͣ�[Zn(OH)4]2-�Ľṹ����ʾ��ͼ��ʾΪ ��ij��ZnO����ľ�����ͼ1��ʾ��O2������λ��Ϊ


ͼ1 ZnO���� ͼ2 GaN����
��4��ͼ2�ǵ����صľ���ģ�͡�������ԭ�ӵ��ӻ���ʽΪ �ӻ���N��Gaԭ��֮�������λ��������λ�����ṩ���ӶԵ�ԭ���� ��������Ϊ���������������߳�Ϊa pm���������ص��ܶ�Ϊ��g��cm��3�����ؾ����߳��ı���ʽa�� pm(��NA��ʾ�����ӵ�������ֵ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ���������ֵ������˵����ȷ����(����)
A. ���³�ѹ�£�8g O2����4NA������
B. 1L 0.1mol��L-1�İ�ˮ����0.1NA��NH4+
C. ��״���£�22.4L���Ȼ�̼����NA������
D. 1molNa����ȫ��������Na2O2��ʧȥ2NA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��װ����ͼ1����Ҫ�������£�
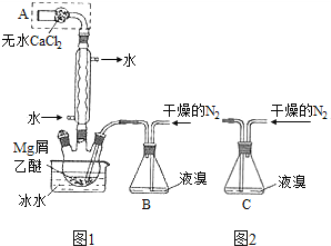
����1 ����ƿ��װ��10 gþм��150 mL��ˮ���ѣ�װ��B�м���15 mLҺ�塣
����2 ����ͨ�����ĵ�����ֱ������ȫ��������ƿ�С�
����3 ��Ӧ��Ϻ�ָ������£����ˣ���Һת������һ�������ƿ�У���ȴ��0�����������壬�ٹ��˵������Ѻ��廯þ��Ʒ��
����4 �������ñ��ܽ��Ʒ����ȴ��0�����������壬���ˣ�ϴ�ӵ������Ѻ��廯þ��������160 ���ֽ����ˮMgBr2��Ʒ��
��֪����Mg��Br2��Ӧ���ҷ��ȣ�MgBr2����ǿ��ˮ�ԡ�
��MgBr2+3C2H5OC2H5![]() MgBr2��3C2H5OC2H5
MgBr2��3C2H5OC2H5
��ش�
��1������A��������____________��ʵ���в����ø�������������N2��ԭ����___________��
��2���罫װ��B��Ϊװ��C(ͼ2)�����ܻᵼ�µĺ����___________��
��3������3�У���һ�ι��˳�ȥ��������___________��
��4���йز���4��˵������ȷ����___________��
A������95%���Ҵ����汽�ܽ��Ʒ
B��ϴ�Ӿ����ѡ��0���ı�
C��������160������ҪĿ���dz�ȥ��
D���ò����Ŀ���dz�ȥ���ѺͿ��ܲ�������
��5��Ϊ�ⶨ��Ʒ�Ĵ��ȣ�����EDTA(��дΪY4-)����Һ�ζ�����Ӧ�����ӷ���ʽ��
Mg2++Y4-=MgY2-
���ζ�ǰ��ϴ�ζ��ܵIJ���������__________��
���ⶨǰ���ȳ�ȡ0.2500g��ˮMgBr2��Ʒ���ܽ����0.0500 mol��L-1��EDTA����Һ�ζ����յ㣬����EDTA����Һ26.50 mL��������ˮMgBr2��Ʒ�Ĵ�����__________________(������������ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ʵ�������������������ʵ��Ӧ�ã������±���������һ���б����ĵ�����������գ�
ʵ�� | ���� |
(1)���������� | ���� |
(2)��������Ũ������ | |
(3)��ʳ���ؽ������ӣ����������ȴ�����ţ�������ⶾ�� | |
(4)��������Һ�м����������͵��������Һ���ֳ��� | |
(5))���þƾ���ϴ�˿� | |
(6)����ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�
CH3CH2CH2CH2OH![]() CH3CH2CH2CHO
CH3CH2CH2CHO
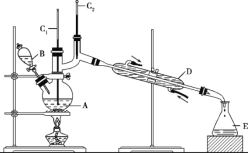
��Ӧ��Ͳ������������б����£�
�е�/��c | �ܶ�/(g��cm-3) | ˮ���ܽ��� | |
������ | 11.72 | 0.8109 | �� |
����ȩ | 75.7 | 0.8017 | �� |
ʵ�鲽�����£�
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90 ~ 95��C����E���ռ�90��C���µ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75 ~ 77��C��֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������_______________________��
��2�������ʯ��������_____________�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������__________________��
��3������װ��ͼ�У�B������������_________________��D������������___________________��
��4����Һ©��ʹ��ǰ������еIJ�����_____________(����ȷ�𰸱��)��
A����ʪ B������ C����© D���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ��____________��(����������������)
��6����Ӧ�¶�Ӧ������90~95��C����ԭ����__________________________��
��7����ʵ���У�����ȩ�IJ���Ϊ___________%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£�1���ˮ�ܽ�672���������������Һ�ܶ�Ϊ0.9g��cm��3�����ְ�ˮ�����ʵ���Ũ�Ⱥ����ʵ����������ֱ�Ϊ( ��
A��17.9mol��L��1 34.7% B��20.4mol��L��1 33.8%
C��17.9mol��L��1 33.8% D��20.4mol��L��1 34.7%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й������ࡢ�����ʡ���֬��ά���ص�˵���У���ȷ����( )
A�����ǵ���ɶ�����C��H��O��N��Ԫ��
B����һ�������£����Ƕ��ܷ���ˮ�ⷴӦ
C��������Ҫ�Ķ�ʮ���ְ����ᣬ������ͨ�����������ϳ�
D����֬���Ǹ�֬����ĸ���������һ�ָ�����Ӫ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com