

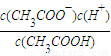 ���Դ˼��㣮
���Դ˼��㣮 ClO-+H2����
ClO-+H2���� ClO-+H2����
ClO-+H2����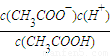 ��c��H+��=10-7mol/L��[CH3COO-]=[Na+]=0.005mol/L��c��CH3COOH��=
��c��H+��=10-7mol/L��[CH3COO-]=[Na+]=0.005mol/L��c��CH3COOH��=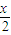 mol/L����Ka=
mol/L����Ka=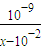 ���ʴ�Ϊ��=
���ʴ�Ϊ��=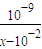 ��
��

 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��ׯ��ģ��������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��
��2012?��ׯ��ģ��������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��
| ||
| ||
| 10-9 |
| x-10-2 |
| 10-9 |
| x-10-2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и�����ѧ�ڵ�һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
������ˮ��Һ���в�ͬ����Ϊ����Ҫ��ش��������⣺
��1��Na2SO3��Һ�Լ��ԣ���ԭ����______________________________(�����ӷ���ʽ��ʾ)������Һ�и�����Ũ���ɴ�С��˳��Ϊ______________________��
��2����������10mL��ˮ��Һ�м�ˮϡ�ͺ��������������__________(���ţ���ͬ)����С����___________���������____________��
a����Һ������Ũ��? ??????? b����ˮ�ĵ���̶�
c��ˮ�����ӻ�����????????? d��c(H+)/ c(NH3��H2O)
��3���������ƣ�NaClO2����һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ��������������δ������NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ���??????????????? ��������ͬ����������ͬ��������ԭ����??????????????????????????????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ׯ��ģ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com