
���и���ʵ�������������Ľ�������ȷ����
A��ij����Һ�м���NaOH��Һ�а�ɫ�������֣���ԭ��Һ��һ������Mg2��
B��ij��Һ�м���AgNO3��Һ�а�ɫ�������֣���ԭ��Һ��һ������Cl��
C��ij��Һ�м������������ʹ����ʯ��ˮ����ǵ���ɫ��ζ�����壬��ԭ��Һ��һ������CO32-��HCO3���е�һ��
D��ij����Һ�м���NaOH��Һ�����ȣ��ܲ���ʹʪ�����ɫʯ����ֽ�������壬��ԭ��Һ��һ������NH4��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʵ����ѧ��һ��10�½β⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��1mol��L��1���Ȼ�����Һ��ָ����Һ�к���1molNaCl
B����1L0��5mol��L��1��NaCl��Һ��ȡ��100mL��Һ�������ʵ���Ũ�ȱ�Ϊ0��1mol��L��1
C��Ħ������ָ1mol���ʵ������ܺͣ���gΪ��λ
D����g��mol��1Ϊ��λʱ������ֵ��Ħ����������Է������������ԭ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵���������Դ������
A����������μӵĻ�ѧ��Ӧ������ѹǿ����ϵ�����С����ʹ��λ����ڻ���������ӣ������Ӧ��������
B�������֮�䷢������ײ��һ��Ϊ��Ч��ײ
C�������¶ȣ�һ���ʹ����ӵİٷ������������Ӧ��������
D���������˵Ĵ�����ʹ�����������ӴӶ���ʹ����ӵİٷ���������ӣ��Ӷ���ǧ��������Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵڶ��μ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A�������ܽ���ˮ��Cl2+H2O ��HClO+Cl-+H+
B����ˮ�е��뱥��FeCl3 ��Һ����Һ�ʺ��ɫ��Fe3++3H2O�� Fe��OH��3��+3H+
C����������Һ�Լ��ԣ�CH3COO-+H2O ��CH3COOH+OH����
D�����õ�H2S ��Һ����ǣ�2S2-+O2+4H+ ��2S��+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ijͬѧ��ʵ���ҽ�������ͼ��ʾ��ʵ�飬����˵���д������
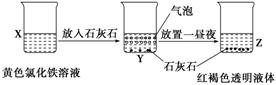
A�����ù��˵ķ������ɽ�Z�й�����Һ�����
B��X��Z�ձ��з�ɢ����ͬ
C��Y�з�Ӧ�����ӷ���ʽΪ��3CaCO3��2Fe3����3H2O===2Fe��OH��3�����壩��3CO2����3Ca2��
D��Z�з�ɢϵ�ܲ��������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���Ϻ��и�����ѧ�����ײ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л���Ľṹ��ͼ:����ͬ���칹�������ڷ��������
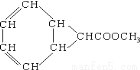
A��8�� B��10�� C��12�� D��14��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꼪��ʡ�߶������п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��pH��Ϊ2������ʹ�����Һ���ֱ��к͵�����������ʵ���Ũ�ȵ�����������Һ������������ǡ�ñ���ȫ�к�ʱ����������ʹ�����Һ������ֱ�ΪVl��V2����Vl��V2�Ĺ�ϵ��ȷ����
A��V1>V2 B��V1<V2 C��V1=V2 D��V1��V2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���Ĵ�������ѧ��һ��10�½β⻯ѧ�Ծ��������棩 ���ͣ������
��10 �֣���1����״�����Т�0��112 L ˮ��0��5NA��HCl���Ӣ�25��6 g SO2�����0��2 mol������2 mol������6��02�� 1023 ������ ��P4�����ӣ�����ԭ�Ӹ����Ӵ�С��˳��Ϊ____________________��
��2����ͬ�¡�ͬѹ�£�ʵ���� CO��N2��O2��������Ļ��������ܶ��� H2��14��5��������O2����������Ϊ____________________��������CO��N2�����ʵ���֮��Ϊ 1��1��������������Ԫ�ص���������Ϊ__________________�������Է�����ʾ��
��3��������Ͽ�ѧ���о��������������ᾧˮ�ľ����� 5 K �³��ֳ����ԡ� �ݱ����� �þ���Ļ�ѧʽΪNa0��35CoO2��1��3H2O�������Ħ������Ϊ 122 g��mol-1���� ���� NA ��ʾ�����ӵ��������Լ��� 12��2 g �þ����к���ԭ����______________����ԭ�ӵ����ʵ���____________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ�ϵ�һ�ζο���ѧ�Ծ��������棩 ���ͣ������
��10�֣���0��4 L��NaCl ��MgCl2 ��CaCl2��ɵĻ��Һ�У���������Ũ�ȴ�С��ͼ��ʾ���ش��������⡣
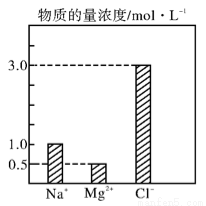
��1���û��Һ�У�NaCl�����ʵ���Ϊ____________mol��������MgCl2������Ϊ______________g��
��2���û��Һ��CaCl2�����ʵ���Ϊ____________mol�����û��Һ��ˮϡ�������Ϊ1 L��ϡ�ͺ���Һ��Ca2�������ʵ���Ũ��Ϊ______________mol��L��1��
��3�����ϡ�ͺ����Һ�м������������ữ����������Һ���ɵõ�����____________mol��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com