
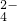 ��=0.8 mol?L-1����c��K+��Ϊ______
��=0.8 mol?L-1����c��K+��Ϊ______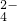 ��������
�������� =1.5mol������ˮ���2L����Һ���ʰ�ˮ�����ʵ���Ũ��Ϊ
=1.5mol������ˮ���2L����Һ���ʰ�ˮ�����ʵ���Ũ��Ϊ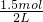 =0.75mol/L��
=0.75mol/L��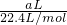 =
=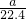 mol�����е���ԭ����Ϊb����
mol�����е���ԭ����Ϊb����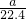 mol��3��NA=b�����NA=
mol��3��NA=b�����NA=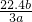 mol-1��
mol-1��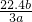 mol-1��
mol-1��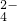 �����ݴ˼��㣻
�����ݴ˼��㣻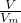 ����33.6L��NH3�����ʵ������ٸ���c=
����33.6L��NH3�����ʵ������ٸ���c= ���㰱ˮ�����ʵ���Ũ�ȣ�
���㰱ˮ�����ʵ���Ũ�ȣ�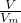 ����aL��NH3�����ʵ�������������Hԭ�ӵ����ʵ������ٸ���N=nNA���㰢��٤��������
����aL��NH3�����ʵ�������������Hԭ�ӵ����ʵ������ٸ���N=nNA���㰢��٤��������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2- 4 |
| 22.4b |
| 3a |
| 22.4b |
| 3a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| 2-4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ÷����÷�ض�ɽ��ѧ��һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
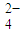 ��=0.8 mol?L-1����c��K+��Ϊ
��=0.8 mol?L-1����c��K+��Ϊ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06��ȫ����1��������ء���������������ɵĻ����Һ����pH��1��c(Al3��)��0.4mol?L��1��c(SO42��)��0.8mol?L��1����c(K��)Ϊ����
A 0.15mol?L��1 B 0.2mol?L��1 C 0.3mol?L��1 D 0.4mol?L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com