
����Ŀ��A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������AԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ��C�ǵؿ��к�������Ԫ�ء�D��E�ǵ�������Ԫ�أ�����EԪ�صĺ˵����Ϊ29��Dԭ�Ӻ���δ�ɶԵ�������ͬ��������ࡣ���ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ�
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
(2)B���⻯����Һ����ԭ���� ��
(3)��֪A��C�γɵĻ�������Ӽ��������ЧӦ��l mol���к�����������ĿΪ ��
��4����̬Dԭ�ӵ���Χ�����Ų�ʽΪ ��DO2Cl2�۵㣺��96 .5�����е㣺 117�������̬DO2Cl2���� ���塣

��5��E���⻯��ľ����ṹ��ͼ��ʾ���仯ѧʽ�� ��
���𰸡���1��C��O��N����2��NH3���Ӽ����γ�������е�ߣ� ��3��1.204��1024����2NA����4��3d54s1���� ����5��CuH��
�����������������A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������AԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ��A��CԪ�أ�C�ǵؿ��к�������Ԫ�أ�C��OԪ�أ�B��NԪ�أ�D��E�ǵ�������Ԫ�أ�����EԪ�صĺ˵����Ϊ29��E��CuԪ�أ�Dԭ�Ӻ���δ�ɶԵ�������ͬ��������࣬D�ļ۵����Ų�ʽΪ3d54s1, ��1��C��N��O�ĵ�һ��������С�����˳��ΪC��O��N����Ϊ��C��O��N��(2 ) N���⻯����Һ����ԭ����NH3���Ӽ����γ�������е�ߣ���Ϊ��NH3���Ӽ����γ�������е�ߣ���3��( 3 )��֪C��O�γɵĻ�������Ӽ��������ЧӦ�����Ӽ���CO2��1��CO2�����к���2��C=O˫��������l mol���к�����������ĿΪ2mol����Ϊ��1.204��1024����2NA ����4��Dԭ�Ӻ���δ�ɶԵ������ڵ�����������࣬D�ļ۵����Ų�ʽΪ3d54s1��CrO2Cl2�۵㣺��96 .5�����е㣺 117����CrO2Cl2���۷е㶼�ܵͣ����Թ�̬DO2Cl2���ڷ��Ӿ��壬��Ϊ��3d54s1���� ����5��Cu�ľ����ṹ��ͼ������ԭ�Ӱ뾶�жϣ�Cuλ�ڶ�������ģ�Cuԭ�ӵĸ���Ϊ8��1/8+6��1/2=4��Hԭ��λ�ھ����ڣ������Ϊ��4�����Ըþ���Ļ�ѧʽΪCuH����Ϊ��CuH.


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������й�ͭԪ�ص�ʵ��������ͼ��

��1��д����̬ͭԭ�ӵļ۵����Ų�ʽ________________________��������ɫ��Һ�м��������Ҵ�������������ɫ���壬�þ����д��ڵĻ�ѧ����������________________________��
��2��д����Ӧ�������ӷ���ʽ________________________��
��3��ͭ������±��(SCN) 2��Ӧ����Cu(SCN) 2��1 mol (SCN)2�����к��е��ļ���ĿΪ��±��(SCN)2��Ӧ���������֣������Ʋ�������(H-S-C![]() N)�ķе������������(H-N=C=S)�ķе㣬��ԭ����__________________��д��һ����SCN-��Ϊ�ȵ�����ķ���_________________(�û�ѧʽ��ʾ)��
N)�ķе������������(H-N=C=S)�ķе㣬��ԭ����__________________��д��һ����SCN-��Ϊ�ȵ�����ķ���_________________(�û�ѧʽ��ʾ)��
��4����������ͭ�ķ��ӽṹ��ͼ��̼ԭ�ӵ��ӻ���ʽΪ____________________��

��5��ͭ������,�侧��Ķѻ���ʽΪ_______________��һ��������ͭԭ�ӵ���ĿΪ__________���ռ�������Ϊ____________��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯��������������������ϢϢ��أ��ش��������⣺
��1����̬Nԭ���е�����2p����ϵ��Ų���ѭ��ԭ����__________��ǰ4����Ԫ���У���̬ԭ�Ӻ�������Ų��ɵ�����������Ԫ�صļ۲�����Ų�ʽΪ__________��
��2��C��N��O����Ԫ�ص�һ�����ܴӴ�С��˳����__________��
��3��N2F2������Nԭ�ӵ��ӻ���ʽ��__________��l mol N2F2����__________mol������
��4��NF3�ļ���__________NH3�ļ���(������������������=��)��ԭ����__________.
��5��NH4BF4(�������)�Ǻϳɵ��������ܵ�ԭ��֮һ��l mol NH4BF4__________mol��λ����
��6����ȫ���ҵ����ԭ��Ϊ6NaN3+FeIO3![]() Na2O+2Fe+9N2��
Na2O+2Fe+9N2��
���ȵ������ԭ���ǣ�ԭ��������ͬ���۵���������ͬ�ķ��ӻ����Ӿ������ƵĻ�ѧ������������������������ʣ�д��������N3-��Ϊ�ȵ�����ķ��ӻ�����__________.
��Na2O�ľ����ṹ��ͼ��ʾ��Ʒ���߳�Ϊ566pm����������ԭ�ӵ���λ��Ϊ__________��Na2O������ܶ�Ϊ__________gcm-3(ֻҪ������ʽ�����ؼ�������)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��50 mL 0.50 mol��L-1��������50 mL 0.55 mol��L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ�����������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��_____________����ָ��װ���е���������� ��
��2���ձ���������ֽ����������___________��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ___________������ƫ������ƫС������Ӱ��������
��4��ʵ���и���60mL0.50mol��L-1�������60mL0.55mol��L-1NaOH��Һ��������ʵ����ȣ����ų�������___________(���������������������������к���___________������������������������
��5������ͬŨ������İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��_____����50 mL 0.50 mol��L-1NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��_____��������ƫ������ƫС������Ӱ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D���ֽ������±���װ�ý���ʵ�顣
װ�� |
|
|
|
���� | ���۽���A �����ܽ� | C���� ������ | A������ ����� |
����ʵ������ش��������⣺
��1��װ�ü��и����ĵ缫��Ӧʽ��____________________________________��
��2��װ�����������ĵ缫��Ӧʽ��_____________________________________����Һ��Cu2����______���ƶ�(����B������C��)��
��3����װ�ñ�������3.36L����״��������ʱ����·��ת�Ƶĵ�����ĿΪ____________��
��4�����ֽ��������ǿ������˳����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������[Al��NO3��3]��һ�ֳ���ýȾ������ҵ�������ң���Ҫ��Al��Al2O3��Fe2O3�ȣ���ȡ����������[Al��NO3��39H2O]��������ͼ�ף�
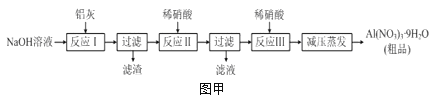
��1��д����Ӧ�������ӷ���ʽ��______________________________________________��
��2������ͼ����ʾʵ��װ����ȡAl��NO3��3��ͨ��ˮ������������__________________��
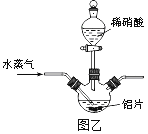
��3�����������в��ü�ѹ�����������Ʒ�Ӧ���м����ϡ�����Թ�������Ŀ����__________��
��4���¶ȸ���200��ʱ����������ȫ�ֽ����������ij���塣��֪��2NO2+2NaOH=NaNO2+NaNO3��Ϊ��ȷ����������ijɷ֣�ijѧ����������װ�ý���ʵ�顣
������ʵ��ʱװ���ӿ���ȷ˳����A��________________________��F��
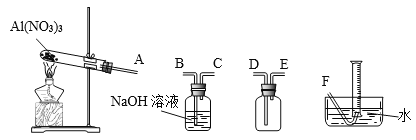
��֤���ֽ����ɵĻ����������NO2��������_____________________��
��ȡ21��3g Al��NO3��3���ȷֽ⣬����ˮ���ռ����壬�����ڱ�״�����ռ���_____mL���壬�����ѧ����ʽ˵������____________________________��
��5�����ʵ��֤����Ʒ�к���Al��NO3��3��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�־���ս������Ľ����������ж��ּ�̬�����ʸ��۵�Ϊ1857 ����
��1����ҵ���Ը��������Ҫ�ɷ���Fe��CrO2��2��Ϊԭ��ұ������������ͼ��ʾ��
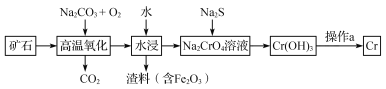
��Fe��CrO2��2�и�Ԫ�ػ��ϼ۾�Ϊ���������Ϊ_______�ۡ�
����������ʱ��Ӧ�Ļ�ѧ����ʽΪ___________________________________��
������a�����־������˻�ѧ��Ӧ�Ĺ��̹��ɵģ������ݷֱ���_______________�����ȷ�Ӧ��
��2��Cr��OH��3���������������д����ֱ���NaOH��ϡ���ᷴӦʱ���ɵ������εĻ�ѧʽ____________________________��
��3����Ԫ�����γɺ����ἰ�������Σ�����ó�ʼŨ��Ϊ1 mol/L�ĸ��� ��H2CrO4�� ��Һ�и��ֺ���Ԫ�ص���Ũ�ȷֱ�Ϊ��c��![]() ��=0.0005 mol��L1��c��
��=0.0005 mol��L1��c��![]() ��=0.1035 mol��L1��c��
��=0.1035 mol��L1��c��![]() ��=a mol��L1����a=______��KHCrO4��Һ��c��OH��___c��H+�������������<����=������
��=a mol��L1����a=______��KHCrO4��Һ��c��OH��___c��H+�������������<����=������
��4��ˮ�еĸ�Ԫ����ˮ�ʼ������������ص������ã������������������
����������![]() ����ˮ����ͨ��Ϊ���������缫�����ˮ��
����ˮ����ͨ��Ϊ���������缫�����ˮ��![]() �����������ɵ����ӻ�ԭ��ΪCr3+�����ɵ�Cr3+�����������ɵ�OH�������Cr��OH��3������ȥ���������ϵĵ缫��ӦʽΪ_________________________����Ҫ������10 mol
�����������ɵ����ӻ�ԭ��ΪCr3+�����ɵ�Cr3+�����������ɵ�OH�������Cr��OH��3������ȥ���������ϵĵ缫��ӦʽΪ_________________________����Ҫ������10 mol ![]() ����ˮ����������Ҫ���ĵ���Ϊ_______g��
����ˮ����������Ҫ���ĵ���Ϊ_______g��
��ת��Ϊ��Ҫ��Ʒ��������������CrxFeyOz��������![]() ����ˮ�м������������ἰ��������������ַ�Ӧ����ͨ��������������������Fe2+��������NaOH���Ϳ���ʹ������Ԫ��ȫ��ת��Ϊ�����������塣д��
����ˮ�м������������ἰ��������������ַ�Ӧ����ͨ��������������������Fe2+��������NaOH���Ϳ���ʹ������Ԫ��ȫ��ת��Ϊ�����������塣д��![]() �����������±�Fe2+��ԭΪCr3+ �����ӷ���ʽ��____________________________________����������1 mol
�����������±�Fe2+��ԭΪCr3+ �����ӷ���ʽ��____________________________________����������1 mol![]() �������������������� ����ˮʱǡ������10 mol FeSO4��������������n��Fe2+����n��Fe3+����3��2ʱ����������Ļ�ѧʽΪ__________��
�������������������� ����ˮʱǡ������10 mol FeSO4��������������n��Fe2+����n��Fe3+����3��2ʱ����������Ļ�ѧʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ƶ�һ�֡����������ͳɱ������ͭ����ȼ�ϵ�ء��õ��ͨ��һ�ָ��ӵ�ͭ��ʴ����������������зŵ����Ϊ2Li+Cu2O+H2O��2Cu��2Li++2OH��������˵������ȷ���ǣ� ��

A���ŵ�һ��ʱ����Ҳ�ˮ��ҺpH����
B��������Ӧ�����У�ͭ�൱�ڴ���
C��ͨ����ʱ��ͭ����ʴ���������CuO
D���ŵ�ʱ�������ĵ缫��ӦʽΪCu2O+H2O+2e����2Cu+2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����X��Y��Z��T��U���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ��������ʾ����Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ����������T2X(��ɫ����)��T2X2(����ɫ����)���ֻ����U������Z������ȼ��ʱ������ɫ���棬�������ˮ��Һ��ʹʯ����Һ��졣
X | |
Y | Z |
��1����Ԫ�صķ����ǣ�Z________��T________
��2��Yԭ�ӵĽṹʾ��ͼΪ____________________,U2X�ĵ���ʽ
��3��YX2��U2Y��Ӧ�Ļ�ѧ����ʽΪ____________________________��������������____________����������Ԫ����____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com