
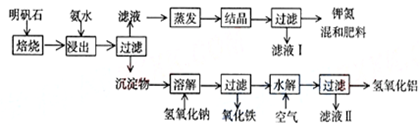
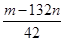
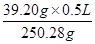 ��0.078L=78mL���ʴ�Ϊ��78��100��
��0.078L=78mL���ʴ�Ϊ��78��100��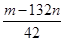 mol��
mol��

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
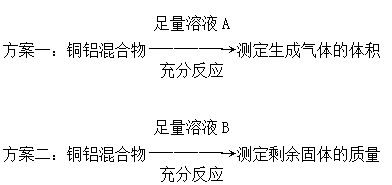
| A����ҺA����ҺB�������������NaOH��Һ |
| B������ҺBѡ��Ũ���ᣬ���ͭ����������ƫ�� |
| C������һ���ܲ�������������������ʣ��ͭ |
| D��ʵ�����з����������ʵʩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
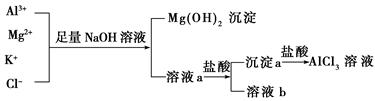
| A��NaOH��Һ�����ð�ˮ������ |
| B����Һaֻ����Al3����K����Cl����Na����OH�� |
| C����Һb��ֻ����NaCl |
| D������Һa�еμ������������Һ��pH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ˮ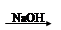 Mg��OH��2 Mg��OH��2 Mg Mg |
B����ˮ MgCl2��Һ��MgCl2���� MgCl2��Һ��MgCl2���� Mg Mg |
C����ˮ Mg��OH��2 Mg��OH��2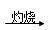 MgO MgO Mg Mg |
D����ˮ Mg��OH��2 Mg��OH��2 MgCl2��Һ��MgCl2���� MgCl2��Һ��MgCl2���� Mg Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
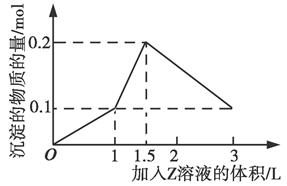
| A��AlCl3��FeCl3��NaOH |
| B��AlCl3��MgCl2��NaOH |
| C��NaAlO2��Ba(OH)2��H2SO4 |
| D��NaAlO2��BaCl2��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ� �ⶨ������������ʵ��װ�ã�
�ⶨ������������ʵ��װ�ã�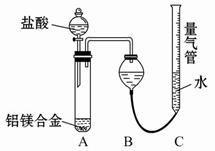

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
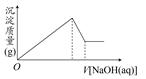
| A����Mg2����û��Al3�� |
| B����Al3����û��Mg2�� |
| C�������H����Mg2����Al3�� |
| D����Mg2����Al3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cr2O3 | B��MnO2 | C��MgO | D��V2O5 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com