
| n(CO) |
| n(H2) |
| n(CO) |
| n(H2) |
 ��д������A�Ļ�ѧ����ʽ��
��д������A�Ļ�ѧ����ʽ�� ����ȷ�����壬Ȼ�������Ŀ����Ϣ�������⣮
����ȷ�����壬Ȼ�������Ŀ����Ϣ�������⣮| ���� |
| n(CO) |
| n(H2) |
| 2 |
| 3 |
| 2 |
| 3 |
| n(CO) |
| n(H2) |
| 2m |
| (2m+n) |
| 2m |
| (2m+n) |
 ���γ����ָ߾���ĵ�����CH3COOCH�TCH2�����Ի�ѧ����ʽΪ��2CO+H2+2CH3OH��CH3COOCH�TCH2+2H2O���ʴ�Ϊ��2CO+H2+2CH3OH��CH3COOCH�TCH2+2H2O��
���γ����ָ߾���ĵ�����CH3COOCH�TCH2�����Ի�ѧ����ʽΪ��2CO+H2+2CH3OH��CH3COOCH�TCH2+2H2O���ʴ�Ϊ��2CO+H2+2CH3OH��CH3COOCH�TCH2+2H2O��


 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��500mL1mol/LFe2��SO4��3��Һ��250mL3mol/L��Na2SO4��Һ������������ӵ����ʵ���Ũ����� |
| B����Ϊ����H2SO3��H2CO3�����Էǽ�����S��C |
| C��1.2gNaHSO4�����������Ӻ������ӵ�����Ϊ0.03NA |
| D���ֱ�Ϊ7.8g��Na2S��Na2O2�к��е����������ֱ�Ϊ0.1NA����0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1 1 |
2 1 |
3 1 |
| A���ڢ� | B���ڢۢ� |
| C���ڢܢ� | D��ȫ����ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
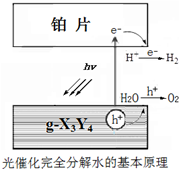 ԭ������������������ֶ�����Ԫ��X��Y��Z��W���������Ϣ���±���
ԭ������������������ֶ�����Ԫ��X��Y��Z��W���������Ϣ���±���| Ԫ�� | �����Ϣ |
| X | X��һ��ͬ����������һ�������Ŀ������������缫 |
| Y | Y���⻯�ﳣ���³���̬����ˮ��Һ��ʹ��̪��Һ��� |
| Z | Z������������ˮ������ۻ�״̬�µ�����������͵��������ȵ��������� |
| W | W�Ļ�̬ԭ�ӵ�ÿ��ԭ�ӹ�����е�����䣬��ֻ��һ��δ�ɶԵ��� |
| 1 |
| 2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 -CH2CH3
-CH2CH3| ����KMn0��Һ |
 +HNO3
+HNO3
| ||
| 55��60�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ˮ�ķ�Ӧ��C12+H2O�T2H++Cl��+ClO�� |
| B��̼��������Һ�м������������CO32-+2H+�T�TCO2��+H2O |
| C��̼������Һ��ͨ�������̼��CO32-+CO2+H2O�T2HCO3- |
| D��������þ�����ᷴӦ��H++OH-�TH2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ɱ��ƻ����顢�������������� |
| B����ϩʹ��ˮ��ɫ����ϩʹ���Ը������ˮ��Һ��ɫ |
| C������ϩ�ƾ���ϩ��ʯ���ͷֽ���ϩ�� |
| D���ɱ������������ɱ����屽 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com