
����ͼװ���У�b�缫�ý��� M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�
��1��aΪ ����c���ĵ缫��ӦʽΪ ��
��2��������һ��ʱ�������c���ϵ��Թ���Ҳ���ռ��������壬��ʱc���ϵĵ缫��ӦʽΪ ��
��3����d�����ռ���44.8mL���壨��״����ʱֹͣ��⣬a���Ϸų�����������ʵ���Ϊ ����b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ ��
��1������1�֣��� 2I��һ2e����I2��2�֣�
��2��40H����4e����2H2O+O2�� ��2�֣�
��3��0��001 mol��1�֣���108g��mol ��2�֣�
��������������ɵ��ԭ���ɵã�����M������b����˵��b����������a��������c��������d����������1����a����������Һ�е������ӷŵ磬�������ӵķŵ�˳��֪��2I--2e-=I2����2����B�ձ��У� c����������Һ�е������ӷŵ磬��2I--2e-=I2��I2����������ʹ���۱�����I-�ŵ���Ϻ�����OH-�ŵ磺4OH--4e=2H2O+O2����c���ϵ��Թ����ռ���������Ϊ��������3��d�缫���ռ���44.8ml���壨��״������������a�����ռ���������������������ת�Ƶ��������֪�����������������֮����1��2��d�缫���ռ���44.8ml������������a�缫���ռ���22.4mL���������ʵ���Ϊ0.01mol��d�缫�����������������ʵ���=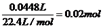 ��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=
��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=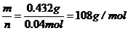
���㣺ԭ��غ͵��صĹ���ԭ����


 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е�����,��Ʋ�����������һϵ��ʵ��,ʵ������¼����:
| ��� | �缫���� | �������Һ | ������ָ�� ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH��Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D��Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2�������ΪKOH��Һ��ij�о�С�齫����ȼ�ϵ����Ϊ��Դ�����Ȼ�þ��Һ���ʵ�飬���װ����ͼ��ʾ��
��ش��������⣺
��1������ȼ�ϵ�ظ����ĵ缫��ӦʽΪ�� ��
��2���պϿ���K��a��b�缫�Ͼ����������������a�缫�ϵ�������� ���飬b�缫�ϵõ��������� ������Ȼ�þ��Һ�����ӷ���ʽΪ ��
��3��������ͨ����Ϊ1.12 L����״�������ҷ�Ӧ��ȫ����������ͨ�����صĵ��ӵ����ʵ���Ϊ ���������������Ϊ L����״������
��4����֪���³�ѹ�£�0.25molCH4��ȫȼ������CO2��H2Oʱ���ų�222.5kJ��������д��CH4ȼ���ȵ��Ȼ�ѧ����ʽ ��
��֪����C��ʯī��+O2��g��=CO2��g����H1=-393��5kJ/mol
��2H2��g��+O2��g��=2H2O��l����H2=-571��6kJ/mol
���㣺C��ʯī����H2��g����Ӧ����1molCH4��g���ġ�H= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ��ijͬѧ�����һ��ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ����������װ����XΪ�����ӽ���Ĥ���밴Ҫ��ش��������:
��1������ȼ�ϵ�ظ����缫��Ӧʽ��:
��2��ʯī�缫(C)�ĵ缫��ӦʽΪ
��3�����ڱ�״���£���2�� 24 L�����μӷ�Ӧ������װ�������缫�����ɵ��������Ϊ_ L;��װ������������ͭ������Ϊ g
��4��ijͬѧ������ȼ�ϵ����Ƶ�ⷨ��ȡƯ��Һ��Fe(OH)2��ʵ��װ��(��ͼ��ʾ)����������Ư��ҺʱaΪ���_ �����������Һ�����_ ���������� Fe(OH)2��ʹ�����������������Һ������ѡ�� ���缫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ��װ���У�����ԭ��ص��� ��
��1����ͼ��ʾ���ձ���ΪCuCl2��Һ����ͼ�л�����Ҫ������װ�ã�ʹ���Ӻ��װ��Ϊԭ��ء��缫��Ӧ����ʽ��
���壺 ��̼���� ��
��2��ͭƬ��пƬ���Ӻ����ϡ�����й���ԭ��أ���������ͨ��3.01��1022������ʱ��пƬ��������________g��ͭƬ������������_________L(��״��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
��ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ����
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(14��)2013������������������Ű������ȵ��������У�ȼú������β����
��ɿ�����Ⱦ��ԭ��֮һ��
(l)����β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g) 2CO2(g)+ N2(g)��H <0
2CO2(g)+ N2(g)��H <0
�ٸ÷�Ӧƽ�ⳣ������ʽ____________________________
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________________������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol ��
2NO2(g)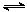 N2O4(g) ��H����56.9 kJ/mol ��
N2O4(g) ��H����56.9 kJ/mol ��
H2O(g)��H2O(l) ��H����44.0 kJ/mol ��
д��CH4����ԭN2O4(g)����N2��H2O��1�����Ȼ�ѧ����ʽ��_____________________��
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��100mLlmol/Lʳ��ˮ�����һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩��
�ټ���ȼ�ϵ�صĸ�����Ӧʽ��______________________________________.
�ڵ�����Һ��pH=____����������������������Һ��Ӧ��
�������������������ڱ�״������________L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪̼̼�������Ƽ���������ת,ij���Ľṹ��ʽ����ͼ��ʾ,����˵������ȷ����
| A������������ԭ�Ӿ��ɹ��� |
| B��������������10��̼ԭ�Ӵ���ͬһƽ���� |
| C��������������11��̼ԭ�Ӵ���ͬһƽ���� |
| D�������뱽��Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com