
ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е�����,��Ʋ�����������һϵ��ʵ��,ʵ������¼����:
| ��� | �缫���� | �������Һ | ������ָ�� ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH��Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
��1������ͬ�� ��2��������2Al-6e-=2Al3+������6H++6e-=3H2���ܷ�Ӧ��2Al+6H+=2Al3++3H2����
��3��������1�֣� ����ΪAl�ܺ�NaOH��Һ��Ӧ��Mg���ܣ�2�֣�
���������������1��ʵ��1�У�������ԭ��Ӧ�����ڽ���þ��ϡ����֮�䣬ʧ���ӵ��ǽ���þ��������������������ʵ��2�У�������ԭ��Ӧ�����ڽ�������ϡ����֮�䣬ʧ���ӵ��ǽ���������������������ʵ��1��2��Al�����ĵ缫����ͬ����2��Al��ʯī�����ṹ�ɵ�ԭ����У��ϻ��õĽ��������������缫��ӦΪ��2Al-6e-��2Al3+��ʯī�缫Ϊ�������缫��ӦΪ��6H++6e-��3H2�����ܷ�Ӧ��2Al+6H+=2Al3++3H2������3��ʵ��4�У����������������Ʒ���������ԭ��Ӧ��ʧ���ӵ��ǽ�������Ϊԭ��صĸ�����
���㣺����ԭ���ԭ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijС�鰴ͼ1��ʾ��װ��̽������������ʴ��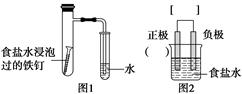
���������գ�
��1��ͼ2��ͼ1��ʾװ�õ�ʾ��ͼ����ͼ2��С��������д�������ϵĻ�ѧʽ���ڷ��������ü�ͷ��ʾ�����������ķ���
��2��д������������Ӧ�ķ���ʽ��
������________________��������________________��
��3����ͼ1װ��ʵ�飬Լ8���Ӳſ���������Һ�����������д�ʩ���Ը���������ع۲쵽Һ����������____________________(����ĸ���)��
a���ô����������֧�Թ��ڵĿ���
b����ʳ��ˮ���ݹ���������պȡ���ۺ�̿�۵Ļ����
c����ëϸ����ܴ��沣�����ܣ������Թܵ�ˮ�еμ�������īˮ
��4�������¶ȿ��Լӿ컯ѧ��Ӧ���ʣ������þƾ��Ƽ��Ⱦ�֧�Թܡ���һ��ʩ________(����С����С�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.������ʳ����ȷ��ҩ�������彡������Ҫ��֤��
����������������Aʳ�Σ�BС�մ�Cƻ��֭��D�����ǣ�E��ù�أ��밴����Ҫ�����(�����)��
����ά����C���� ����ֱ�ӽ���ѪҺ�������������� ��Ӧ����㷺�Ŀ�����֮һ���� ���ȿ���Ϊ���ɼ����ֿ�����θ�������� ��ʳ�ù��������Ѫѹ���ߡ���������� ��
��.������������ʹ�����������������������һ����Ҫ��־��
��1��д����ҵ���ó�������������Ҫ��ѧ��Ӧ����ʽ�� ��
��2�������˵�����δ��ʱϴ������Һ�к�NaCl��,�ڶ�������ʴ���ֺ��ɫ��ߡ��Իش�
�������ĸ�ʴ��Ҫ���� ���ѧ��绯ѧ����ʴ��ɵġ��γɵ��������Ҫ�ɷ��� ��
��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ������� ���п�顱��ͭ�顱����
��.������������������ͷ�չ����Ҫ���ʻ���������ʹ�ò��Ͽ��Ը������ǵ����
��1���������ݽ���������������ϡ����в��ϲ����ڹ����β��ϵ��� ������ĸ����
A��ʯ��ʯ B��ˮ�� C������
��2�������в����У��������ǽ������ϵ��� (����ĸ)������������Ʒ���� ��
A�����ڡ������� B��������ϩ���ϡ���C���������մ� D��������
��3�������йغϽ����ʵ�˵����ȷ���� ������ĸ����
A���Ͻ���۵�һ������ijɷֽ�����
B���Ͻ��Ӳ��һ������ijɷֽ�����
C����ɺϽ��Ԫ��������ͬ���Ͻ�����ܾ�һ����ͬ
D���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е����
��4���ϳ����ϡ��ϳ��� �dz�˵������ϳɲ��ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ�Ĺ���������ʾ��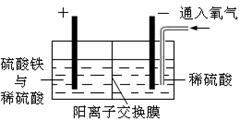
����ͭ����Ҫ�ɷ�ΪCuFeS2����������CaO��MgO��Al2O3�����顣
������ͼ��ʾװ�ý��е绯ѧ����ʵ�顣����ѡ��ͭ��ۼ�����۵������������ٽ��裬ʹ����ܽ⡣��������ͨ������������������������
��һ��ʱ���ȡ��������Һ�������м����л���ȡ��(RH)������Ӧ��2RH(�л���)+Cu2+(ˮ��) R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
��1����ͭ��ۼ��������������ἰ��������Ҫ�������·�Ӧ��CuFeS2 + 4H+��Cu2+ + Fe2+ + 2H2S�� 2Fe3+ + H2S��2Fe2+ + S��+ 2H+ ������������������Ҫ������ ����2�����������缫�Ͽ�ʼʱ�д������ݲ��������к�ɫ����������һ��ʱ����ɫ�����ܽ⡣д��������ɫ����ķ�Ӧ����ʽ ��
��3������ʵ���ҽ��в�������л����ˮ�����Ҫʵ�������� ��
��4����������л����м���һ��Ũ�ȵ����ᣬCu2+����������ԭ���� ����5��������������200mL0.5 mol/L��CuSO4��Һ������ͭ3.2 g����ʱ��Һ������Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������һ�����Ϳɳ���أ�����ͨ���ܵ����ȣ��õ�س�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ3Zn��2K2FeO4��8H2O 3Zn��OH��2��2Fe��OH��3��4KOH����Ҫ��ش��������⣺
3Zn��OH��2��2Fe��OH��3��4KOH����Ҫ��ش��������⣺
��1���ŵ�ʱ�� _______�������ʻ�ѧʽ����ͬ�������������ʱ��____________��������
��2���ŵ�ʱ����������Һ�ļ���__________ �����ǿ����������
��3�����ʱ��ÿת��3mol���ӣ����������ʵ����ʵ���Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش��������⣺
��1����ҵ�ϲ��õ���������ͱ���ʯ(Na3AlF6)������ķ���ұ���õ���������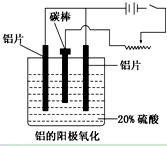
2Al2O3  4Al��3O2��
4Al��3O2��
�������ʯ�����ã�________________________________________________��
��2��������������������������������Fe��Si�����ʣ����õ�ⷽ����һ���ᴿ���õ����������ĵ缫��ӦʽΪ________________�����п����������ϵ���__________��
A������ B��ʯī C��Ǧ�� D������
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ�������������ĵ缫��ӦʽΪ_____________________________________________________________��
��4�������������������У���Ҫ���ϵص�����ѹ��������_________________��
��5������˵����ȷ����__________________��
A������������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B������������������ǿ������ľ�Ե����
C������������������߽���������Ͻ����ʴ�ԣ�����ĥ���½�
D��������������Ĥ���ж���ԣ����к�ǿ���������ܣ�������Ⱦ�϶��ʸ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ��װ�á���֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH=2K2CO3+6H2O����ش�
��ͨ��O2�ĵ缫������ ��B�缫�������� ��
��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ�� ��A�缫�ĵ缫��ӦʽΪ ��
���ҳ����Ϊ1L����AgNO3����������µ��һ��ʱ�����Һ��PH��Ϊ1���������ʱ����ת�Ƶĵ�����ĿΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�ֿ��Է�����硢�ŵ��װ�á���һ�������ڳ��ͷŵ�ʱ�����ķ�ӦΪNiO2+Fe+2H2O Fe(OH)2+Ni(OH)2
Fe(OH)2+Ni(OH)2
��1�������طŵ�ʱ��������ԭ��Ӧ�������� ������ĸ����ͬ����
A��NiO2 B��Fe C��Fe(OH)2 D��Ni(OH)2
��2�������йظõ�ص�˵������ȷ����
A���ŵ�ʱ�������Һ��ǿ����
B���ŵ�ʱ5.6g Feȫ��ת��ΪFe(OH)2ʱ�����·��ͨ����0.2 mol����
C�����ʱ������ӦΪNi(OH)2+2OH��?2e��==NiO2+2H2O
D�����ʱ����������Һ�ļ��Ա��ֲ���
��3���ô����ص�⺬��0.01 mol CuSO4��0.01 mol NaCl�Ļ����Һ100 mL�����صĵ缫��Ϊ���Ե缫������Һ�е�Cu2+ ȫ��ת����Cuʱ�����������������ڱ�״���µ����Ϊ �����������Һ��ˮϡ����1L����ʱ��Һ��pH= ��
��4���ô����ؽ��е�⣬�ҵ��صĵ缫��Ϊͭ�缫���������ҺΪŨ��Һ��NaCl��Һ�Ļ��Һ�����һ��ʱ���ͬѧ�Ǿ���ط��֣�������������������ɫ�������������ɺ�ɫ�������������ϵ�֪�ú�ɫ������Cu2O��д���������ϵĵ缫��Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼװ���У�b�缫�ý��� M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�
��1��aΪ ����c���ĵ缫��ӦʽΪ ��
��2��������һ��ʱ�������c���ϵ��Թ���Ҳ���ռ��������壬��ʱc���ϵĵ缫��ӦʽΪ ��
��3����d�����ռ���44.8mL���壨��״����ʱֹͣ��⣬a���Ϸų�����������ʵ���Ϊ ����b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com