
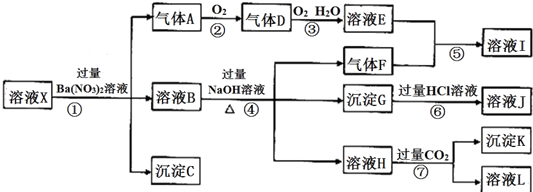
���� ǿ������Һ��һ���������CO32-��SO32-���ӣ�����������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��A������������D��E������DΪ����ɫ����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ���ҺB�м������NaOH��Һ����������F����FΪNH3��˵����Һ�к���NH4+���ӣ���ҺIΪNH4NO3����ҺH��ͨ�������CO2���壬���ɳ���K�����������ṩ�����ӿ�֪����KΪAl��OH��3��HΪNaAlO2��˵����Һ�к���Al3+���ӣ���ҺLΪNaHCO3������GΪFe��OH��3����ҺJΪFeCl3����Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-���Դ������
��� �⣺ǿ������Һ��һ���������CO32-��SO32-���ӣ�����������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��A������������D��E������DΪ����ɫ����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ���ҺB�м������NaOH��Һ����������F����FΪNH3��˵����Һ�к���NH4+���ӣ���ҺIΪNH4NO3����ҺH��ͨ�������CO2���壬���ɳ���K�����������ṩ�����ӿ�֪����KΪAl��OH��3��HΪNaAlO2��˵����Һ�к���Al3+���ӣ���ҺLΪNaHCO3������GΪFe��OH��3����ҺJΪFeCl3����Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-��
��1��������������֪����ҺX��һ�������ڵ�������CO32-��SO32-��NO3-��Ba2+�����ܺ�Fe3+��Cl-��Ҫ������Һ���Ƿ���Cl-����ȡX��Һ�������Թ��У��������������ᱵ��Һ�����˺�����Һ�м���������Һ����ϡ���ᣬ���а�ɫ��Һ���֣����֤����Cl-���ڣ�
�ʴ�Ϊ��CO32-��SO32-��NO3-��Ba2+�� ȡX��Һ�������Թ��У��������������ᱵ��Һ�����˺�����Һ�м���������Һ����ϡ���ᣬ���а�ɫ��Һ���֣����֤����Cl-���ڣ�
��2�������������A�����ӷ���ʽΪ3Fe2++4H++NO3-�T3Fe3++NO��+2H2O��
��������ɳ���K�� ���ӷ���ʽΪAlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
�ʴ�Ϊ��3Fe2++4H++NO3-�T3Fe3++NO��+2H2O��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
��3��AΪNO��FΪNH3��KΪAl��OH��3��A��F��K��Ϊ0.01mol��10mLX��Һ��n��H+��=0.04mol�����ݷ�Ӧ3Fe2++NO3-+4H+=3Fe3++NO��+2H2O����֪��������Ϊ0.03mol��������Һ�����Կ�֪��n��H+��+2n��Fe2+��+3n��Al3+��+n��NH4+��=2n��SO42-����n��SO42-��=$\frac{0.04+2��0.03+3��0.01+0.01}{2}$=0.07mol��������C���ʵ���С��0.07molʱ����n��SO42-��С��0.07mol������Һ��һ������һ�������ӣ�������Һ�в���ȷ��������ֻ��һ�֣�ӦΪCl-���ӣ��ʴ�Ϊ��Cl-��
��4��ͨ����������KClO��һ������������G�Ʊ�һ�����͡���Ч�����ˮ������K2FeO4���Ʊ������е����ӷ���ʽΪ3ClO-+2Fe��OH��3+4OH-�T3Cl-+2FeO42-+5H2O���ʴ�Ϊ��3ClO-+2Fe��OH��3+4OH-�T3Cl-+2FeO42-+5H2O��
���� ���⿼���������ƶ��⣬��Ŀ����һ���Ѷȣ�������ʱһ��Ҫ���۷�Ӧ�����ƶϸ����Ӵ��ڵĿ����ԣ������״���Ϊ��3���⣬ע����Һ�����Ե����ã�


 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1L 0.5mol•L-1 NH4NO3��Һ�к���ԭ����Ŀ6.02��1023 | |
| B�� | ��״���£�22.4L��C3H8����C-H������ĿΪ6.02��1023 | |
| C�� | 32g SO2������O2��ϳ�ַ�Ӧ��ת�Ƶĵ�����ĿΪ6.02��1023 | |
| D�� | �����£�500mL pH=0��������Һ�к��е�H+��ĿΪ6.02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | b-a=n+m | B�� | a-b=n+m | C�� | �˵����X��Y | D�� | ������Y��X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ | B�� | Na2CO3��Һ | C�� | NaHSO3��Һ | D�� | KI��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��aΪŨ���ᣬbΪNa2SO3���壬c��ʢ��ɫʯ����Һ����c����Һ��� | |
| B�� | ��aΪ������Һ��bΪ���ǣ�c��ʢ��������ʯ��ˮ����c����Һ����� | |
| C�� | ��aΪŨ���ᣬbΪMnO2��c��ʢƷ����Һ����c����Һ��ɫ | |
| D�� | ��aΪŨ��ˮ��bΪ��ʯ�ң�c��ʢAlCl3��Һ����c�в�����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
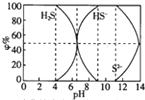 25��ʱ0.1mol/L�����ƺ�S��ݦ�%�������ʵ������㣩�ֲ���ͼ���£����н�����ȷ���ǣ�������
25��ʱ0.1mol/L�����ƺ�S��ݦ�%�������ʵ������㣩�ֲ���ͼ���£����н�����ȷ���ǣ�������| A�� | ��c��HS-����c��S2-��ʱ����Һһ�������� | |
| B�� | ��pH=7ʱ����Һ����c��Na+��=c��HS-��+2c��S2-��+c��H2S�� | |
| C�� | ��4��pHʱ�������еμ�0.1mol/LCuSO4����CuS������Ksp��CuS��=6.3��10-36�� | |
| D�� | ��pH=9ʱ����Һ����c��H+��=c��OH-��+c��HS-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com